N,N-Dimethyltryptamine (DMT)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

Long-Lasting Analgesic Effect of the Psychedelic Drug Changa: a Case Report
CASE REPORT Journal of Psychedelic Studies 3(1), pp. 7–13 (2019) DOI: 10.1556/2054.2019.001 First published online February 12, 2019 Long-lasting analgesic effect of the psychedelic drug changa: A case report GENÍS ONA1* and SEBASTIÁN TRONCOSO2 1Department of Anthropology, Philosophy and Social Work, Universitat Rovira i Virgili, Tarragona, Spain 2Independent Researcher (Received: August 23, 2018; accepted: January 8, 2019) Background and aims: Pain is the most prevalent symptom of a health condition, and it is inappropriately treated in many cases. Here, we present a case report in which we observe a long-lasting analgesic effect produced by changa,a psychedelic drug that contains the psychoactive N,N-dimethyltryptamine and ground seeds of Peganum harmala, which are rich in β-carbolines. Methods: We describe the case and offer a brief review of supportive findings. Results: A long-lasting analgesic effect after the use of changa was reported. Possible analgesic mechanisms are discussed. We suggest that both pharmacological and non-pharmacological factors could be involved. Conclusion: These findings offer preliminary evidence of the analgesic effect of changa, but due to its complex pharmacological actions, involving many neurotransmitter systems, further research is needed in order to establish the specific mechanisms at work. Keywords: analgesic, pain, psychedelic, psychoactive, DMT, β-carboline alkaloids INTRODUCTION effects of ayahuasca usually last between 3 and 5 hr (McKenna & Riba, 2015), but the effects of smoked changa – The treatment of pain is one of the most significant chal- last about 15 30 min (Ott, 1994). lenges in the history of medicine. At present, there are still many challenges that hamper pain’s appropriate treatment, as recently stated by American Pain Society (Gereau et al., CASE DESCRIPTION 2014). -

EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011)
EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011) 912 final COMMISSION STAFF WORKING PAPER on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances Accompanying the document REPORT FROM THE COMMISSION on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances {COM(2011) 430 final} EN EN TABLE OF CONTENTS 1. Introduction...................................................................................................................3 2. Methodology.................................................................................................................4 3. Key findings from the 2002 evaluation of the Joint Action on synthetic drugs ...........5 4. Overview of notifications, types of substances and trends at EU level 2005-2010......7 5. Other EU legislation relevant for the regulation of new psychoactive substances.....12 6. Functioning of the Council Decision on new psychoactive substances .....................16 7. Findings of the survey among Member States............................................................17 7.1. Assessment of the Council Decision ..........................................................................17 7.2. Stages in the functioning of the Council Decision .....................................................18 7.3. National responses to new psychoactive substances ..................................................20 -

Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics
TESI DOCTORAL HUMAN PHARMACOLOGY OF AYAHUASCA JORDI RIBA Barcelona, 2003 Director de la Tesi: DR. MANEL JOSEP BARBANOJ RODRÍGUEZ A la Núria, el Marc i l’Emma. No pasaremos en silencio una de las cosas que á nuestro modo de ver llamará la atención... toman un bejuco llamado Ayahuasca (bejuco de muerto ó almas) del cual hacen un lijero cocimiento...esta bebida es narcótica, como debe suponerse, i á pocos momentos empieza a producir los mas raros fenómenos...Yo, por mí, sé decir que cuando he tomado el Ayahuasca he sentido rodeos de cabeza, luego un viaje aéreo en el que recuerdo percibia las prespectivas mas deliciosas, grandes ciudades, elevadas torres, hermosos parques i otros objetos bellísimos; luego me figuraba abandonado en un bosque i acometido de algunas fieras, de las que me defendia; en seguida tenia sensación fuerte de sueño del cual recordaba con dolor i pesadez de cabeza, i algunas veces mal estar general. Manuel Villavicencio Geografía de la República del Ecuador (1858) Das, was den Indianer den “Aya-huasca-Trank” lieben macht, sind, abgesehen von den Traumgesichten, die auf sein persönliches Glück Bezug habenden Bilder, die sein inneres Auge während des narkotischen Zustandes schaut. Louis Lewin Phantastica (1927) Agraïments La present tesi doctoral constitueix la fase final d’una idea nascuda ara fa gairebé nou anys. El fet que aquest treball sobre la farmacologia humana de l’ayahuasca hagi estat una realitat es deu fonamentalment al suport constant del seu director, el Manel Barbanoj. Voldria expressar-li la meva gratitud pel seu recolzament entusiàstic d’aquest projecte, molt allunyat, per la natura del fàrmac objecte d’estudi, dels que fins al moment s’havien dut a terme a l’Àrea d’Investigació Farmacològica de l’Hospital de Sant Pau. -

(DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact
pharmaceuticals Review Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact Andreia Machado Brito-da-Costa 1 , Diana Dias-da-Silva 1,2,* , Nelson G. M. Gomes 1,3 , Ricardo Jorge Dinis-Oliveira 1,2,4,* and Áurea Madureira-Carvalho 1,3 1 Department of Sciences, IINFACTS-Institute of Research and Advanced Training in Health Sciences and Technologies, University Institute of Health Sciences (IUCS), CESPU, CRL, 4585-116 Gandra, Portugal; [email protected] (A.M.B.-d.-C.); ngomes@ff.up.pt (N.G.M.G.); [email protected] (Á.M.-C.) 2 UCIBIO-REQUIMTE, Laboratory of Toxicology, Department of Biological Sciences, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal 3 LAQV-REQUIMTE, Laboratory of Pharmacognosy, Department of Chemistry, Faculty of Pharmacy, University of Porto, 4050-313 Porto, Portugal 4 Department of Public Health and Forensic Sciences, and Medical Education, Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal * Correspondence: [email protected] (D.D.-d.-S.); [email protected] (R.J.D.-O.); Tel.: +351-224-157-216 (R.J.D.-O.) Received: 21 September 2020; Accepted: 20 October 2020; Published: 23 October 2020 Abstract: Ayahuasca is a hallucinogenic botanical beverage originally used by indigenous Amazonian tribes in religious ceremonies and therapeutic practices. While ethnobotanical surveys still indicate its spiritual and medicinal uses, consumption of ayahuasca has been progressively related with a recreational purpose, particularly in Western societies. The ayahuasca aqueous concoction is typically prepared from the leaves of the N,N-dimethyltryptamine (DMT)-containing Psychotria viridis, and the stem and bark of Banisteriopsis caapi, the plant source of harmala alkaloids. -

A General Approach to the Screening and Confirmation of Tryptamines And
Available online at www.sciencedirect.com Talanta 74 (2008) 512–517 A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation Bo-Hong Chen a, Ju-Tsung Liu b, Wen-Xiong Chen a, Hung-Ming Chen a, Cheng-Huang Lin a,∗ a Department of Chemistry, National Taiwan Normal University, 88 Sec. 4, Tingchow Road, Taipei, Taiwan b Forensic Science Center, Military Police Command, Department of Defense, Taipei, Taiwan Received 6 March 2007; received in revised form 12 June 2007; accepted 13 June 2007 Available online 19 June 2007 Abstract Certain characteristic fragmentations of tryptamines (indoleethylamine) and phenethylamines are described. Based on the GC–EI/MS, LC–ESI/ MS and MALDI/TOFMS, the mass fragmentations of 13 standard compounds, including ␣-methyltryptamine (AMT), N,N-dimethyltryptamine (DMT), 5-methoxy-␣-methyltryptamine (5-MeO-AMT), N,N-diethyltryptamine (DET), N,N-dipropyltryptamine (DPT), N,N-dibutyltryptamine (DBT), N,N-diisopropyltryptamine (DIPT), 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), 5-methoxy-N,N-diisopropyltryptamine (5-MeO- DIPT), methamphetamine (MAMP), 3,4-methylenedioxyamphetamine (3,4-MDA), 3,4-methylenedioxymethamphetamine (3,4-MDMA) and 2- methylamino-1-(3,4-methylenedioxyphenyl)butane (MBDB), were compared. As a result, the parent ions of these analytes were hard to be obtained by GC/MS whereas the protonated molecular ions can be observed clearly by means of ESI/MS and MALDI/TOFMS. Furthermore, two major + + + + characteristic fragmentations, namely and ␣-cleavage ([M +H] → [3-vinylindole] ) and -cleavage ([M +H] → [CH2N RN1RN2]), are produced when the ESI and MALDI modes are used, respectively. In the case of ESI/MS, the fragment obtained from ␣-cleavage is the major process. -

PAPER Monoamine Oxidase Inhibition Is Unlikely to Be Relevant To
International Journal of Obesity (2001) 25, 1454–1458 ß 2001 Nature Publishing Group All rights reserved 0307–0565/01 $15.00 www.nature.com/ijo PAPER Monoamine oxidase inhibition is unlikely to be relevant to the risks associated with phentermine and fenfluramine: a comparison with their abilities to evoke monoamine release{ IC Kilpatrick1*, M Traut2 and DJ Heal1 1Knoll Limited Research and Development, Nottingham, UK; and 2Knoll GmbH, 50 Knollstrasse, D-67061, Ludwigshafen, Germany OBJECTIVE AND DESIGN: It has been proposed that the anti-obesity agent, phentermine, may act in part via inhibition of monoamine oxidase (MAO). The ability of phentermine to inhibit both MAOA and MAOB in vitro has been examined along with that of the fenfluramine isomers, a range of selective serotonin reuptake inhibitors and sibutramine and its active metabolites. RESULTS: In rat brain, harmaline and lazabemide showed potent and selective inhibition of MAOA and MAOB, their respective target enzymes, with IC50 values of 2.3 and 18 nM. In contrast, all other drugs examined were only weak inhibitors of MAOA and MAOB with IC50 values for each enzyme in the moderate to high micromolar range. For MAOA, the IC50 for phentermine was estimated to be 143 mM, that for S( þ )-fenfluramine, 265 mM and that for sertraline, 31 mM. For MAOB, example IC50s were as follows: phentermine (285 mM), S( þ )-fenfluramine (800 mM) and paroxetine (16 mM). Sibutramine was unable to inhibit either enzyme, even at its limit of solubility. CONCLUSION: We therefore suggest that MAO inhibition is unlikely to play a role in the pharmacodynamic properties of any of the tested drugs, including phentermine. -

LSD), Which Produces Illicit Market in the USA
DEPENDENCE LIABILITY OF "NON-NARCOTIC 9 DRUGS 81 INDOLES The prototype drug in this subgroup (Table XVI) potentials. Ibogaine (S 212) has appeared in the is compound S 219, lysergide (LSD), which produces illicit market in the USA. dependence of the hallucinogen (LSD) type (see above). A tremendous literature on LSD exists which documents fully the dangers of abuse, which REFERENCES is now widespread in the USA, Canada, the United 304. Sandoz Pharmaceuticals Bibliography on Psychoto- Kingdom, Australia and many western European mimetics (1943-1966). Reprinted by the US countries (for references see Table XVI). LSD must Department of Health, Education, & Welfare, be judged as a very dangerous substance which has National Institute of Mental Health, Washington, no established therapeutic use. D.C. 305. Cerletti, A. (1958) In: Heim, R. & Wasson, G. R., Substances S 200-S 203, S 206, S 208, S 213-S 218 ed., Les champignons hallucinogenes du Mexique, and S 220-S 222 are isomers or congeners of LSD. pp. 268-271, Museum national d'Histoire natu- A number of these are much less potent than LSD in relle, Paris (Etude pharmacologique de la hallucinogenic effect or are not hallucinogenic at all psilocybine) (compounds S 203, S 213, S 216, S 217, S 220 and 306. Cohen, S. (1965) The beyond within. The LSD S 222) and accordingly carry a lesser degree of risk story. Atheneum, New York than LSD. None of these weak hallucinogens has 307. Cohen, S. & Ditman, K. S. (1963) Arch. gen. been abused. Other compounds are all sufficiently Psychiat., 8, 475 (Prolonged adverse reactions to potent to make it likely that they would be abused if lysergic acid diethylamide) 308. -

Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss
Supplemental Material can be found at: /content/suppl/2020/12/18/73.1.202.DC1.html 1521-0081/73/1/202–277$35.00 https://doi.org/10.1124/pharmrev.120.000056 PHARMACOLOGICAL REVIEWS Pharmacol Rev 73:202–277, January 2021 Copyright © 2020 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: MICHAEL NADER Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss Antonio Inserra, Danilo De Gregorio, and Gabriella Gobbi Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montreal, Quebec, Canada Abstract ...................................................................................205 Significance Statement. ..................................................................205 I. Introduction . ..............................................................................205 A. Review Outline ........................................................................205 B. Psychiatric Disorders and the Need for Novel Pharmacotherapies .......................206 C. Psychedelic Compounds as Novel Therapeutics in Psychiatry: Overview and Comparison with Current Available Treatments . .....................................206 D. Classical or Serotonergic Psychedelics versus Nonclassical Psychedelics: Definition ......208 Downloaded from E. Dissociative Anesthetics................................................................209 F. Empathogens-Entactogens . ............................................................209 -

Chemical Composition of Traditional and Analog Ayahuasca
Journal of Psychoactive Drugs ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/ujpd20 Chemical Composition of Traditional and Analog Ayahuasca Helle Kaasik , Rita C. Z. Souza , Flávia S. Zandonadi , Luís Fernando Tófoli & Alessandra Sussulini To cite this article: Helle Kaasik , Rita C. Z. Souza , Flávia S. Zandonadi , Luís Fernando Tófoli & Alessandra Sussulini (2020): Chemical Composition of Traditional and Analog Ayahuasca, Journal of Psychoactive Drugs, DOI: 10.1080/02791072.2020.1815911 To link to this article: https://doi.org/10.1080/02791072.2020.1815911 View supplementary material Published online: 08 Sep 2020. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ujpd20 JOURNAL OF PSYCHOACTIVE DRUGS https://doi.org/10.1080/02791072.2020.1815911 Chemical Composition of Traditional and Analog Ayahuasca Helle Kaasik a, Rita C. Z. Souzab, Flávia S. Zandonadib, Luís Fernando Tófoli c, and Alessandra Sussulinib aSchool of Theology and Religious Studies; and Institute of Physics, University of Tartu, Tartu, Estonia; bLaboratory of Bioanalytics and Integrated Omics (LaBIOmics), Institute of Chemistry, University of Campinas (UNICAMP), Campinas, SP, Brazil; cInterdisciplinary Cooperation for Ayahuasca Research and Outreach (ICARO), School of Medical Sciences, University of Campinas (UNICAMP), Campinas, Brazil ABSTRACT ARTICLE HISTORY Traditional ayahuasca can be defined as a brew made from Amazonian vine Banisteriopsis caapi and Received 17 April 2020 Amazonian admixture plants. Ayahuasca is used by indigenous groups in Amazonia, as a sacrament Accepted 6 July 2020 in syncretic Brazilian religions, and in healing and spiritual ceremonies internationally. -
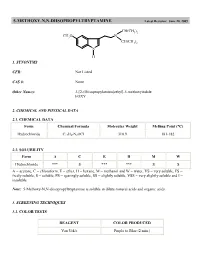
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

5/1 (2005) 41 - 4541
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by idUS. Depósito de Investigación Universidad de Sevilla Vol. 5/1 (2005) 41 - 4541 JOURNAL OF NATURAL REMEDIES Cytotoxic activity of methanolic extract and two alkaloids extracted from seeds of Peganum harmala L. Hicham Berrougui1,2*, Miguel López-Lázaro1, Carmen Martin-Cordero1, Mohamed Mamouchi2, Abdelkader Ettaib2, Maria Dolores Herrera1. 1. Department of Pharmacology School of Pharmacy. Séville, Spain. 2. School of Medicine and Pharmacy, UFR (Natural Substances), Rabat, Morocco. Abstract Objective: To study the cytotoxic activity of P. harmala. Materials and method: The alkaloids harmine and harmaline have been isolated from a methanolic extract from the seeds of P. harmala L. and have been characterized by spectroscopic-Mass and NMR methods. The cytotoxicity of the methanolic extract and both alkaloids has been investigated in the three human cancer cell lines UACC-62 (melanoma), TK- 10 (renal) and MCF-7 (breast) and then compared to the positive control effect of the etoposide. Results and conclusion: The methanolic extract and both alkaloids have inhibited the growth of these three cancer cell-lines and we have discussed possible mechanisms involved in their cytotoxicity. Keywords: Peganum harmala, harmine, harmaline, cytotoxicity, TK-10, MCF-7, UACC-62. 1. Introduction Peganum harmala L. (Zygophyllaceae), the so- convulsive or anticonvulsive actions and called harmal, grows spontaneously in binding to various receptors including 5-HT uncultivated and steppes areas in semiarid and receptors and the benzodiazepine binding site pre-deserted regions in south Spain and South- of GABAA receptors [10]. In addition, these East Morocco [1].