LSD), Which Produces Illicit Market in the USA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

Determination of Sex 43, Elm Park Gardens, THOSE Who Are Interested in the Heredity of Sex Chelsea, S.W.Lo
APRIL 14, 1934 NATURE 579 sa was correctly computed in five minutes, 510 in genes outweigh the female and the result is the twenty seconds and 610 in seventy seconds. normal haplo-X male." Division was a slower process and 9 digits divided Thus, as my italics show, the experimental by 3 took times varying from two and a half to geneticist seems to agree with what Prof. MacBride seven and three quarters minutes. has expressed in more generally intelligible language ; Square roots of 6 digit numbers were extracted in not only in admitting the essential sameness of sex less than a minute while cube roots took longer. in all organisms but also in understanding the Curiously enough, the memorising of a number of function of proportion in its determination in some 27 digits was not done successfully, although he of them. Unanimity among the different branches of could repeat questions which had been put to him biology has therefore been reached after a long period and their answers after some days had elapsed, and of divergence, from entirely different data and, what would break off calculations in the middle to ask for is more, apparently unawares. Such an event, surely, milk or cigarettes, taking up the calculations again should not be allowed to pass without notice and where he had broken off. His methods of working without applause. The usual view that the chromo were not discovered, but he had obviously memorised some theory of sex determination criticised by the squares of two digit numbers, and less completely MacBride was a special hypothesis put forward by the products of two digit numbers. -

HIGHLIGHTS of PRESCRIBING INFORMATION These Highlights Do
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MEMANTINE and DONEPEZIL ---------------------CONTRAINDICATIONS -------------------- HYDROCHLORIDES EXTENDED-RELEASE Memantine and donepezil hydrochlorides extended-release CAPSULES safely and effectively. See full prescribing capsules are contraindicated in patients with known information for MEMANTINE and DONEPEZIL hypersensitivity to memantine hydrochloride, donepezil HYDROCHLORIDES EXTENDED-RELEASE hydrochloride, piperidine derivatives, or to any excipients CAPSULES. used in the formulation. (4) MEMANTINE and DONEPEZIL HYDROCHLORIDES ------------------WARNINGS AND PRECAUTIONS ---------- extended-release capsules, for oral use • Memantine and donepezil hydrochlorides extended- Initial U.S. Approval: 2014 release is likely to exaggerate succinylcholine-type muscle relaxation during anesthesia. (5.1) ------------------RECENT MAJOR CHANGES ----------------- • Memantine and donepezil hydrochlorides extended- Indications and Usage (1) 07/2016 release may have vagotonic effects on the sinoatrial and Dosage and Administration (2.1, 2.3) 07/2016 atrioventricular nodes manifesting as bradycardia or heart block. (5.2) ------------------INDICATIONS AND USAGE------------------ • Monitor patients for symptoms of active or occult Memantine and donepezil hydrochlorides extended-release gastrointestinal bleeding, especially those at increased capsules are a combination of memantine hydrochloride, an risk for developing ulcers. (5.3) NMDA receptor -

EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011)
EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011) 912 final COMMISSION STAFF WORKING PAPER on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances Accompanying the document REPORT FROM THE COMMISSION on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances {COM(2011) 430 final} EN EN TABLE OF CONTENTS 1. Introduction...................................................................................................................3 2. Methodology.................................................................................................................4 3. Key findings from the 2002 evaluation of the Joint Action on synthetic drugs ...........5 4. Overview of notifications, types of substances and trends at EU level 2005-2010......7 5. Other EU legislation relevant for the regulation of new psychoactive substances.....12 6. Functioning of the Council Decision on new psychoactive substances .....................16 7. Findings of the survey among Member States............................................................17 7.1. Assessment of the Council Decision ..........................................................................17 7.2. Stages in the functioning of the Council Decision .....................................................18 7.3. National responses to new psychoactive substances ..................................................20 -

Temporary Class Drug Order Report: 5-6APB and Nbome Compounds
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Rachel Fowler 3rd Floor (SW), Seacole Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 0555 [email protected] Home Secretary Rt Hon. Theresa May MP Home Office 2 Marsham Street London SW1P 4DF 29 May 2013 Dear Home Secretary, I am writing to formally request that you consider laying a temporary class drug order (TCDO) pursuant to section 2A of the Misuse of Drugs Act 1971 on the following two groups of novel psychoactive substances (NPS). We consider the laying of TCDOs is appropriate as a pre-emptive measure in advance of the summer music festival season. Both classes of drugs have been associated with serious harm and drug-related deaths. ‘Benzofury’ compounds 5- and 6-APB and related substances are phenethylamine-type materials, related to ecstasy (MDMA). There have been several deaths and hospitalisations in the UK associated with these NPS, although poly-substance use often complicates the case. Research indicates that there is a potential risk of cardiac toxicity associated with the long-term use of 5- and 6-APB. Anecdotal user reports suggest that the consumption of these substances can cause insomnia, increased heart rate and anxiety, with some users reporting MDMA like symptoms. The related compound 5-IT has been subject to an EMCDDA-Europol joint report and an EMCDDA risk assessment exercise. [www.emcdda.europa.eu/publications/joint-reports/5- IT] 1 The substances recommended for control are: 5- and 6-APB: (1-(benzofuran-5-yl)-propan-2-amine and 1-(benzofuran-6-yl)-propan- 2-amine) and their N-methyl derivatives. -

A General Approach to the Screening and Confirmation of Tryptamines And
Available online at www.sciencedirect.com Talanta 74 (2008) 512–517 A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation Bo-Hong Chen a, Ju-Tsung Liu b, Wen-Xiong Chen a, Hung-Ming Chen a, Cheng-Huang Lin a,∗ a Department of Chemistry, National Taiwan Normal University, 88 Sec. 4, Tingchow Road, Taipei, Taiwan b Forensic Science Center, Military Police Command, Department of Defense, Taipei, Taiwan Received 6 March 2007; received in revised form 12 June 2007; accepted 13 June 2007 Available online 19 June 2007 Abstract Certain characteristic fragmentations of tryptamines (indoleethylamine) and phenethylamines are described. Based on the GC–EI/MS, LC–ESI/ MS and MALDI/TOFMS, the mass fragmentations of 13 standard compounds, including ␣-methyltryptamine (AMT), N,N-dimethyltryptamine (DMT), 5-methoxy-␣-methyltryptamine (5-MeO-AMT), N,N-diethyltryptamine (DET), N,N-dipropyltryptamine (DPT), N,N-dibutyltryptamine (DBT), N,N-diisopropyltryptamine (DIPT), 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), 5-methoxy-N,N-diisopropyltryptamine (5-MeO- DIPT), methamphetamine (MAMP), 3,4-methylenedioxyamphetamine (3,4-MDA), 3,4-methylenedioxymethamphetamine (3,4-MDMA) and 2- methylamino-1-(3,4-methylenedioxyphenyl)butane (MBDB), were compared. As a result, the parent ions of these analytes were hard to be obtained by GC/MS whereas the protonated molecular ions can be observed clearly by means of ESI/MS and MALDI/TOFMS. Furthermore, two major + + + + characteristic fragmentations, namely and ␣-cleavage ([M +H] → [3-vinylindole] ) and -cleavage ([M +H] → [CH2N RN1RN2]), are produced when the ESI and MALDI modes are used, respectively. In the case of ESI/MS, the fragment obtained from ␣-cleavage is the major process. -

Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com
Hallucinogens And Dissociative Drug Use And Addiction Introduction Hallucinogens are a diverse group of drugs that cause alterations in perception, thought, or mood. This heterogeneous group has compounds with different chemical structures, different mechanisms of action, and different adverse effects. Despite their description, most hallucinogens do not consistently cause hallucinations. The drugs are more likely to cause changes in mood or in thought than actual hallucinations. Hallucinogenic substances that form naturally have been used worldwide for millennia to induce altered states for religious or spiritual purposes. While these practices still exist, the more common use of hallucinogens today involves the recreational use of synthetic hallucinogens. Hallucinogen And Dissociative Drug Toxicity Hallucinogens comprise a collection of compounds that are used to induce hallucinations or alterations of consciousness. Hallucinogens are drugs that cause alteration of visual, auditory, or tactile perceptions; they are also referred to as a class of drugs that cause alteration of thought and emotion. Hallucinogens disrupt a person’s ability to think and communicate effectively. Hallucinations are defined as false sensations that have no basis in reality: The sensory experience is not actually there. The term “hallucinogen” is slightly misleading because hallucinogens do not consistently cause hallucinations. 1 ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com How hallucinogens cause alterations in a person’s sensory experience is not entirely understood. Hallucinogens work, at least in part, by disrupting communication between neurotransmitter systems throughout the body including those that regulate sleep, hunger, sexual behavior and muscle control. Patients under the influence of hallucinogens may show a wide range of unusual and often sudden, volatile behaviors with the potential to rapidly fluctuate from a relaxed, euphoric state to one of extreme agitation and aggression. -

Risk Assessment of Argyreia Nervosa
Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos RIVM letter report 2019-0210 Colophon © RIVM 2020 Parts of this publication may be reproduced, provided acknowledgement is given to the: National Institute for Public Health and the Environment, and the title and year of publication are cited. DOI 10.21945/RIVM-2019-0210 W. Chen (author), RIVM L. de Wit-Bos (author), RIVM Contact: Lianne de Wit Department of Food Safety (VVH) [email protected] This investigation was performed by order of NVWA, within the framework of 9.4.46 Published by: National Institute for Public Health and the Environment, RIVM P.O. Box1 | 3720 BA Bilthoven The Netherlands www.rivm.nl/en Page 2 of 42 RIVM letter report 2019-0210 Synopsis Risk assessment of Argyreia nervosa In the Netherlands, seeds from the plant Hawaiian Baby Woodrose (Argyreia nervosa) are being sold as a so-called ‘legal high’ in smart shops and by internet retailers. The use of these seeds is unsafe. They can cause hallucinogenic effects, nausea, vomiting, elevated heart rate, elevated blood pressure, (severe) fatigue and lethargy. These health effects can occur even when the seeds are consumed at the recommended dose. This is the conclusion of a risk assessment performed by RIVM. Hawaiian Baby Woodrose seeds are sold as raw seeds or in capsules. The raw seeds can be eaten as such, or after being crushed and dissolved in liquid (generally hot water). -

Recommended Methods for the Identification and Analysis Of
Vienna International Centre, P.O. Box 500, 1400 Vienna, Austria Tel: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES Laboratory and Scientific Section United Nations Office on Drugs and Crime Vienna RECOMMENDED METHODS FOR THE IDENTIFICATION AND ANALYSIS OF AMPHETAMINE, METHAMPHETAMINE AND THEIR RING-SUBSTITUTED ANALOGUES IN SEIZED MATERIALS (revised and updated) MANUAL FOR USE BY NATIONAL DRUG TESTING LABORATORIES UNITED NATIONS New York, 2006 Note Mention of company names and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. ST/NAR/34 UNITED NATIONS PUBLICATION Sales No. E.06.XI.1 ISBN 92-1-148208-9 Acknowledgements UNODC’s Laboratory and Scientific Section wishes to express its thanks to the experts who participated in the Consultative Meeting on “The Review of Methods for the Identification and Analysis of Amphetamine-type Stimulants (ATS) and Their Ring-substituted Analogues in Seized Material” for their contribution to the contents of this manual. Ms. Rosa Alis Rodríguez, Laboratorio de Drogas y Sanidad de Baleares, Palma de Mallorca, Spain Dr. Hans Bergkvist, SKL—National Laboratory of Forensic Science, Linköping, Sweden Ms. Warank Boonchuay, Division of Narcotics Analysis, Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand Dr. Rainer Dahlenburg, Bundeskriminalamt/KT34, Wiesbaden, Germany Mr. Adrian V. Kemmenoe, The Forensic Science Service, Birmingham Laboratory, Birmingham, United Kingdom Dr. Tohru Kishi, National Research Institute of Police Science, Chiba, Japan Dr. -

Increased Spontaneous EEG Signal Diversity During Stroboscopically
bioRxiv preprint doi: https://doi.org/10.1101/511766; this version posted January 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Increased spontaneous EEG signal diversity during stroboscopically-induced altered states of consciousness Abbreviated title: EEG signal diversity during stroboscopic hallucinations David J. Schwartzman a,b*, Michael Schartner b,c, Benjamin B. Ador d, Francesca Simonelli e, Acer Y.-C. Chang b,f , Anil K. Seth a,b,g a Department of Informatics, University of Sussex, Brighton BN1 9QJ, United Kingdom b Sackler Centre for Consciousness Science, University of Sussex, Brighton BN1 9QJ, United Kingdom c Département des Neurosciences Fondamentales, Université de Genève, 1206, Genève d Master de Biologie, École Normale Supérieure de Lyon, Université Claude Bernard Lyon I, Université de Lyon, 69342, Lyon, Cedex 07, France e IMT School for Advanced Studies, Lucca, Italy f Department of Neuroinformatics, Araya Brain Imaging, Tokyo, Japan g Canadian Institute for Advanced Research, Azrieli Programme in Brain, Mind, and Consciousness *[email protected] bioRxiv preprint doi: https://doi.org/10.1101/511766; this version posted January 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract What are the global neuronal signatures of altered states of consciousness (ASC)? Recently, increases in neural signal diversity, compared to those found in wakeful rest, have been reported during psychedelic states. -
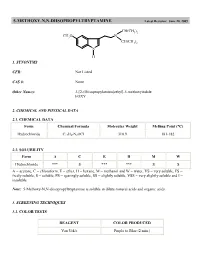
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

Hallucinogens: an Update
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Hallucinogens: An Update 146 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Hallucinogens: An Update Editors: Geraline C. Lin, Ph.D. National Institute on Drug Abuse Richard A. Glennon, Ph.D. Virginia Commonwealth University NIDA Research Monograph 146 1994 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGEMENT This monograph is based on the papers from a technical review on “Hallucinogens: An Update” held on July 13-14, 1992. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company.