Urinary Excretion Profiles of 5-Methoxy-N,N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting
Western Michigan University ScholarWorks at WMU Dissertations Graduate College 6-2020 The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting Robert J. Kohler Western Michigan University, [email protected] Follow this and additional works at: https://scholarworks.wmich.edu/dissertations Part of the Biological Psychology Commons Recommended Citation Kohler, Robert J., "The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting" (2020). Dissertations. 3565. https://scholarworks.wmich.edu/dissertations/3565 This Dissertation-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Dissertations by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THE EFFECTS OF LOW DOSE LYSERGIC ACID DIETHYLAMIDE ADMINISTRATION IN A RODENT MODEL OF DELAY DISCOUNTING by Robert J. Kohler A dissertation submitted to the Graduate College In partial fulfillment of the requirements for the degree of Doctor of Philosophy Psychology Western Michigan University June 2020 Doctoral Committee: Lisa Baker, Ph.D., Chair Anthony DeFulio, Ph.D. Cynthia Pietras, Ph.D. John Spitsbergen, Ph.D. Copyright by Robert J. Kohler 2020 THE EFFECTS OF LOW DOSE LYSERGIC ACID DIETHYLAMIDE ADMINISTRATION IN A RODENT MODEL OF DELAY DISCOUNTING Robert J. Kohler, Ph.D. Western Michigan University, 2020 The resurgence of Lysergic Acid Diethylamide (LSD) as a therapeutic tool requires a revival in research, both basic and clinical, to bridge gaps in knowledge left from a previous generation of work. Currently, no study has been published with the intent of establishing optimal microdose concentrations of LSD in an animal model. -

A General Approach to the Screening and Confirmation of Tryptamines And
Available online at www.sciencedirect.com Talanta 74 (2008) 512–517 A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation Bo-Hong Chen a, Ju-Tsung Liu b, Wen-Xiong Chen a, Hung-Ming Chen a, Cheng-Huang Lin a,∗ a Department of Chemistry, National Taiwan Normal University, 88 Sec. 4, Tingchow Road, Taipei, Taiwan b Forensic Science Center, Military Police Command, Department of Defense, Taipei, Taiwan Received 6 March 2007; received in revised form 12 June 2007; accepted 13 June 2007 Available online 19 June 2007 Abstract Certain characteristic fragmentations of tryptamines (indoleethylamine) and phenethylamines are described. Based on the GC–EI/MS, LC–ESI/ MS and MALDI/TOFMS, the mass fragmentations of 13 standard compounds, including ␣-methyltryptamine (AMT), N,N-dimethyltryptamine (DMT), 5-methoxy-␣-methyltryptamine (5-MeO-AMT), N,N-diethyltryptamine (DET), N,N-dipropyltryptamine (DPT), N,N-dibutyltryptamine (DBT), N,N-diisopropyltryptamine (DIPT), 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), 5-methoxy-N,N-diisopropyltryptamine (5-MeO- DIPT), methamphetamine (MAMP), 3,4-methylenedioxyamphetamine (3,4-MDA), 3,4-methylenedioxymethamphetamine (3,4-MDMA) and 2- methylamino-1-(3,4-methylenedioxyphenyl)butane (MBDB), were compared. As a result, the parent ions of these analytes were hard to be obtained by GC/MS whereas the protonated molecular ions can be observed clearly by means of ESI/MS and MALDI/TOFMS. Furthermore, two major + + + + characteristic fragmentations, namely and ␣-cleavage ([M +H] → [3-vinylindole] ) and -cleavage ([M +H] → [CH2N RN1RN2]), are produced when the ESI and MALDI modes are used, respectively. In the case of ESI/MS, the fragment obtained from ␣-cleavage is the major process. -

21 CFR Ch. II (4–1–10 Edition) § 1308.12
§ 1308.12 21 CFR Ch. II (4–1–10 Edition) Some trade and other names: N,N- whenever the existence of such salts, Diethyltryptamine; DET isomers, and salts of isomers is possible (18) Dimethyltryptamine ................................................. 7435 Some trade or other names: DMT within the specific chemical designa- (19) 5-methoxy-N,N-diisopropyltryptamine (other tion: name: 5-MeO-DIPT) ................................................... 7439 (1) gamma-hydroxybutyric acid (some other names in- (20) Ibogaine .................................................................. 7260 clude GHB; gamma-hydroxybutyrate; 4- Some trade and other names: 7-Ethyl- hydroxybutyrate; 4-hydroxybutanoic acid; sodium 6,6b,7,8,9,10,12,13-octahydro-2-methoxy-6,9- oxybate; sodium oxybutyrate) .................................... 2010 methano-5H-pyrido [1′, 2′:1,2] azepino [5,4-b] (2) Mecloqualone ........................................................... 2572 indole; Tabernanthe iboga (3) Methaqualone ........................................................... 2565 (21) Lysergic acid diethylamide ..................................... 7315 (22) Marihuana .............................................................. 7360 (f) Stimulants. Unless specifically ex- (23) Mescaline ............................................................... 7381 cepted or unless listed in another (24) Parahexyl—7374; some trade or other names: 3- schedule, any material, compound, Hexyl-1-hydroxy-7,8,9,10-tetrahydro-6,6,9-trimethyl- 6H-dibenzo[b,d]pyran; Synhexyl. mixture, -

Hallucinogen-Like Actions of 5-Methoxy-N,N-Diisopropyltryptamine in Mice and Rats ⁎ W.E
Pharmacology, Biochemistry and Behavior 83 (2006) 122–129 www.elsevier.com/locate/pharmbiochembeh Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats ⁎ W.E. Fantegrossi a,b, , A.W. Harrington b, C.L. Kiessel b, J.R. Eckler c, R.A. Rabin c, J.C. Winter c, A. Coop d, K.C. Rice e, J.H. Woods b a Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, GA 30322, United States b Department of Pharmacology, University of Michigan Medical School, Ann Arbor, MI, United States c Department of Pharmacology and Toxicology, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY, United States d Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, MD, United States e Laboratory of Medicinal Chemistry, NIDDK, National Institutes of Health, Bethesda, MD Received 2 June 2005; received in revised form 7 December 2005; accepted 29 December 2005 Available online 3 February 2006 Abstract Few studies have examined the effects of 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT) in vivo. In these studies, 5-MeO-DIPT was tested in a drug-elicited head twitch assay in mice where it was compared to the structurally similar hallucinogen N,N-dimethyltryptamine (N,N- DMT) and challenged with the selective serotonin (5-HT)2A antagonist M100907, and in a lysergic acid diethylamide (LSD) discrimination assay in rats where its subjective effects were challenged with M100907 or the 5-HT1A selective antagonist WAY-100635. Finally, the affinity of 5- MeO-DIPT for three distinct 5-HT receptors was determined in rat brain. -
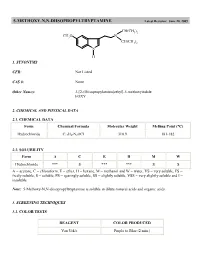
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

M a P S • V O L U M E X I I I N U M B E R 2
m a p s • v o l u m e x i i i n u m b e r 2 • w i n t e r 2 0 0 3 1 2 m a p s • v o l u m e x i i i n u m b e r 2 • w i n t e r 2 0 0 3 Divine Spark The cover of this MAPS bulletin is an image from visionary artist Alex Grey’s triptych Holy Fire, which was inspired by one of Alex’s early MDMA experiences. We thought it was an appropriate choice to ex- press our emotions at obtaining Institutional Review Board (IRB) approval for Dr. Mithoefer's MAPS-sponsored MDMA/PTSD study (see page 7). The rest of the images on this page and on the back cover are from the 2003 Burning Man Festival, at which MAPS provided psychedelic emergency services. (Article on page 28). In the photo above, members of the Fire Conclave open the festivities at the Burning of the Man, the climax of the week-long festival. The photo below depicts the more somber burning of the Temple of Honor, at which Burning Man participants remember loved ones by leaving notes and tokens inside. As the temple burned, MAPS president Rick Doblin threw in the fire the original copies of the last set of documents about protocol design issues exchanged between MAPS and the IRB. This symbolized his hopes that we would finally move beyond the paperwork of the approval process to the therapeutic work of the study itself. -

Hallucinogens: an Update
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Hallucinogens: An Update 146 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Hallucinogens: An Update Editors: Geraline C. Lin, Ph.D. National Institute on Drug Abuse Richard A. Glennon, Ph.D. Virginia Commonwealth University NIDA Research Monograph 146 1994 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGEMENT This monograph is based on the papers from a technical review on “Hallucinogens: An Update” held on July 13-14, 1992. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

Designer Drugs: a Review
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Chavan et al. World Journal of Pharmacy and Pharmaceutical Sciences SJIF Impact Factor 5.210 Volume 4, Issue 08, 297-336. Review Article ISSN 2278 – 4357 DESIGNER DRUGS: A REVIEW Dr. Suyash Chavan,MBBS*1 and Dr. Vandana Roy2 1MD, Resident Doctor, Department of Pharmacology, Maulana Azad Medical College, New Delhi. 2MD, PhD Professor, Department of Pharmacology, Maulana Azad Medical College, New Delhi. ABSTRACT Article Received on 25 May 2015, Designer drugs‟ are psychoactive substances that mimic the effects of Revised on 16 June 2015, other banned illicit drugs but evade detection by law enforcing Accepted on 07 July 2015 agencies. This is because of modifications in the structure of the original psychoactive molecule. Originally developed as a way to *Correspondence for evade existing drug laws in the late 1960s, the synthesis and use of Author designer drugs has increased dramatically. They are advertised with Dr. Suyash Chavan innocuous names and are sold mostly over the internet, discreet outlets MD, Resident Doctor, Department of and at entertainment clubs. Victims may exhibit symptoms similar to Pharmacology, Maulana the effects of the illegal drug that these synthetic drugs mimic, Azad Medical College, however, the exact culprit drug is not detected due to structural New Delhi. modifications in the new drug. Overdose of these drugs may lead to serious adverse effects that can be life threatening. Understanding the pharmacology and toxicology of these agents is essential to facilitate their detection and to provide better medical care for patients suffering from adverse effects due to their consumption. -

N,N-Dimethyltryptamine Compound Found in the Hallucinogenic Tea Ayahuasca, Regulates Adult Neurogenesis in Vitro and in Vivo Jose A
Morales-Garcia et al. Translational Psychiatry (2020) 10:331 https://doi.org/10.1038/s41398-020-01011-0 Translational Psychiatry ARTICLE Open Access N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo Jose A. Morales-Garcia 1,2,3,4, Javier Calleja-Conde 5, Jose A. Lopez-Moreno 5, Sandra Alonso-Gil1,2, Marina Sanz-SanCristobal1,2, Jordi Riba6 and Ana Perez-Castillo 1,2,4 Abstract N,N-dimethyltryptamine (DMT) is a component of the ayahuasca brew traditionally used for ritual and therapeutic purposes across several South American countries. Here, we have examined, in vitro and vivo, the potential neurogenic effect of DMT. Our results demonstrate that DMT administration activates the main adult neurogenic niche, the subgranular zone of the dentate gyrus of the hippocampus, promoting newly generated neurons in the granular zone. Moreover, these mice performed better, compared to control non-treated animals, in memory tests, which suggest a functional relevance for the DMT-induced new production of neurons in the hippocampus. Interestingly, the neurogenic effect of DMT appears to involve signaling via sigma-1 receptor (S1R) activation since S1R antagonist blocked the neurogenic effect. Taken together, our results demonstrate that DMT treatment activates the subgranular neurogenic niche regulating the proliferation of neural stem cells, the migration of neuroblasts, and promoting the generation of new neurons in the hippocampus, therefore enhancing adult neurogenesis and -

Newer Unregulated Drugs Look-Up Table
Newer Unregulated Drugs Look-up Table List Name Chemical Name/AKA Type of drug Notes Stimulant Regulation under MDA (Sch. 1 or TCDO) Stimulant/Hallucinogen Regulation under MDA (Sch. 2-5) Hallucinogen Regulated by PSA Depressant Exempt Cannabinoid Uncertain/requires clarification 1P-LSD 1-propionyl-lysergic acid diethylamide Hallucinogen An LSD analogue that side-stepped MDA and was on sale as an NPS; now covered by the PSA. 2-AI 2-Aminoindane Stimulant, amphetamine analogue Reported in the UK in 2011 by the Forensic Early 2-MAI N-methyl-2-Aminoindane Warning System (FEWS). Had been on sale via number MMAI of online stores; covered by PSA. 2-MeO-ketamine Methoxyketamine Related to methoxetamine so a relative Believed to have been made a CD at the same time as Methoxieticyclidine of ketamine – i.e. a dissassociative Methoxetamine anaesthetic hallucinogen 2C-B-BZP (1-(4-bromo-2,5- Piperazine family; stimulant Class B dimethoxybenzyl)piperazine) 2-DPMP Desoxypipadrol stimulant Strong and long acting stimulant; reported duration of 2-diphenylmethylpiperidine effect 24-28hrs or more and effective at very low doses. Had been on sale in the UK and cropped up in branded “Ivory Wave” and in other compounds. Linked to fatalities. Class B, Sch1. 2-NE1 APICA Synthetic cannabinoid receptor agonist 3rd generation SCRA. Covered by PSA SDB-001 N-(1-adamantyl)-1-pentyl-1H-indole-3- carboxamide 3-FPM Phenzacaine Stimulant, euphoriants Sibling of the controlled drug Phenmetrazine. Emerged PAL-593 2015. Covered by PSA 2-(3-fluorophenyl)-3-methylmorpholine 3-hydroxyphenazepam Benzo, GABA-nergic PSA 3-MeO-PCE (3-methoxyeticyclidine) Related to methoxetamine so a relative Probably regulated under the same clause that made of ketamine – i.e.