CODING: Words Stricken Are Deletions; Words Underlined Are Additions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

Synthetic Cannabinoids-1) IMMUNALYSIS DIRECT ELISA Kit for Forensic Matrices
K2 (Synthetic IMMUNALYSIS Cannabinoids-1) DIRECT ELISA Kit for forensic matrices JWH-018 Demonstrates significant cross reactivity with the major metabolites of JWH-018, JWH-073, Schedule I Controlled Substances: JWH-018, JWH-073, JWH-200, JWH-019, JWH-200 and AM-2201. JWH-122, JWH-398, JWH-081, JWH-250, JWH-203 CP-47,497, CP-47,497 C8, HU-210, HU-211, AM-2201, AM-694, RCS-4 (SR-19), RCS-8 (SR-18) Street Names: Spice, K2, Genie, Yucatan Fire, Skunk, Sence, Smoke, ChillX, Highdi’s Almdröhner, Earth Impact, Gorillaz, Galaxy Gold, Space Truckin, Solar Flare, Moon Rocks, Blue Lotus, Aroma, Scope, Sky, OG Potpourri, Bliss, Black Momba, Bombay Blue, Fake Weed, and Zohai. Urine About Synthetic Cannabinoids: Spice or K2 is a mixture of herbs and spices treated with synthetic compounds similar to THC that is typically sold in head shops, tobacco shops or over the internet. Though not structurally related, K2 mimics the psychoactive stimulant properties of THC but can be 100 to 800 times more potent than THC.1 Administration: Synthetic Cannabinoid products are usually smoked in joints or pipes, and sometimes made into tea.2 Effects: Psychological effects are similar to those of marijuana and include paranoia, panic attacks and giddiness. Physiological effects include increased heart rate and high blood pressure. Long-term effects are not known.2 1. Devane, W. A. et al. A novel probe for the cannabi- noid receptor. Journal of Medical Chemistry 35 (11): 2065–2069 (1992). 2. Drug Enforcement Administration; www.dea.gov. Tel 909.482.0840 | Toll Free -

Federal Register/Vol. 85, No. 178/Monday, September 14, 2020
Federal Register / Vol. 85, No. 178 / Monday, September 14, 2020 / Notices 56631 agreements. All non-confidential DEPARTMENT OF JUSTICE ADDRESSES: Written comments should written submissions will be available for be sent to: Drug Enforcement public inspection at the Office of the Drug Enforcement Administration Administration, Attention: DEA Federal Secretary and on EDIS. [Docket No. DEA–713] Register Representative/DPW, 8701 The Commission vote for these Morrissette Drive, Springfield, Virginia 22152. All requests for a hearing must determinations took place on September Importer of Controlled Substances Application: Cerilliant Corporation be sent to: Drug Enforcement 8, 2020. Administration, Attn: Administrator, The authority for the Commission’s AGENCY: Drug Enforcement 8701 Morrissette Drive, Springfield, determination is contained in section Administration, Justice. Virginia 22152. All request for a hearing 337 of the Tariff Act of 1930, as ACTION: Notice of application. should also be sent to: (1) Drug amended (19 U.S.C. 1337), and in Part SUMMARY: Cerilliant Corporation has Enforcement Administration, Attn: 210 of the Commission’s Rules of applied to be registered as an importer Hearing Clerk/OALJ, 8701 Morrissette Practice and Procedure (19 CFR part of basic class(es) of controlled Drive, Springfield, Virginia 22152; and 210). substance(s). Refer to Supplemental (2) Drug Enforcement Administration, Attn: DEA Federal Register By order of the Commission. Information listed below for further Representative/DPW, 8701 Morrissette Issued: September 8, 2020. drug information. DATES: Drive, Springfield, Virginia 22152. Lisa Barton, Registered bulk manufacturers of the affected basic class(es), and SUPPLEMENTARY INFORMATION: In Secretary to the Commission. applicants therefore, may file written accordance with 21 CFR 1301.34(a), this [FR Doc. -

Synthetic Cannabinoids (60 Substances) A) Classical Cannabinoid
Synthetic cannabinoids (60 substances) a) Classical cannabinoid OH H OH H O Common name Chemical name CAS number Molecular Formula HU-210 3-(1,1’-dimethylheptyl)-6aR,7,10,10aR-tetrahydro-1- Synonym: 112830-95-2 C H O hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol 25 38 3 11-Hydroxy-Δ-8-THC-DMH b) Nonclassical cannabinoids OH OH R2 R3 R4 R1 CAS Molecular Common name Chemical name R1 R2 R3 R4 number Formula rel-2[(1 S,3 R)-3- hydroxycyclohexyl]- 5- (2- methyloctan- 2- yl) CP-47,497 70434-82-1 C H O CH H H H phenol 21 34 2 3 rel-2[(1 S,3 R)-3- hydroxycyclohexyl]- 5- (2- methylheptan- 2- yl) CP-47,497-C6 - C H O H H H H phenol 20 32 2 CP-47,497-C8 rel-2- [(1 S,3 R)-3- hydroxycyclohexyl]- 5- (2- methylnonan- 2- yl) 70434-92-3 C H O C H H H H Synonym: Cannabicyclohexanol phenol 22 36 2 2 5 CAS Molecular Common name Chemical name R1 R2 R3 R4 number Formula rel-2[(1 S,3 R)-3- hydroxycyclohexyl]- 5- (2- methyldecan- 2- yl) CP-47,497-C9 - C H O C H H H H phenol 23 38 2 3 7 rel-2- ((1 R,2 R,5 R)-5- hydroxy- 2- (3- hydroxypropyl)cyclohexyl)- 3-hydroxy CP-55,940 83003-12-7 C H O CH H H 5-(2- methyloctan- 2- yl)phenol 24 40 3 3 propyl rel-2- [(1 S,3 R)-3- hydroxy-5,5-dimethylcyclohexyl]- 5- (2- Dimethyl CP-47,497-C8 - C H O C H CH CH H methylnonan-2- yl)phenol 24 40 2 2 5 3 3 c) Aminoalkylindoles i) Naphthoylindoles 1' R R3' R2' O N CAS Molecular Common name Chemical name R1’ R2’ R3’ number Formula [1-[(1- methyl- 2- piperidinyl)methyl]- 1 H-indol- 3- yl]- 1- 1-methyl-2- AM-1220 137642-54-7 C H N O H H naphthalenyl-methanone 26 26 2 piperidinyl -

21 CFR Ch. II (4–1–10 Edition) § 1308.12
§ 1308.12 21 CFR Ch. II (4–1–10 Edition) Some trade and other names: N,N- whenever the existence of such salts, Diethyltryptamine; DET isomers, and salts of isomers is possible (18) Dimethyltryptamine ................................................. 7435 Some trade or other names: DMT within the specific chemical designa- (19) 5-methoxy-N,N-diisopropyltryptamine (other tion: name: 5-MeO-DIPT) ................................................... 7439 (1) gamma-hydroxybutyric acid (some other names in- (20) Ibogaine .................................................................. 7260 clude GHB; gamma-hydroxybutyrate; 4- Some trade and other names: 7-Ethyl- hydroxybutyrate; 4-hydroxybutanoic acid; sodium 6,6b,7,8,9,10,12,13-octahydro-2-methoxy-6,9- oxybate; sodium oxybutyrate) .................................... 2010 methano-5H-pyrido [1′, 2′:1,2] azepino [5,4-b] (2) Mecloqualone ........................................................... 2572 indole; Tabernanthe iboga (3) Methaqualone ........................................................... 2565 (21) Lysergic acid diethylamide ..................................... 7315 (22) Marihuana .............................................................. 7360 (f) Stimulants. Unless specifically ex- (23) Mescaline ............................................................... 7381 cepted or unless listed in another (24) Parahexyl—7374; some trade or other names: 3- schedule, any material, compound, Hexyl-1-hydroxy-7,8,9,10-tetrahydro-6,6,9-trimethyl- 6H-dibenzo[b,d]pyran; Synhexyl. mixture, -

Article 22 Regulation for Restriction of Synthetic Drugs
ARTICLE 22 REGULATION FOR RESTRICTION OF SYNTHETIC DRUGS SECTION 22.1 AUTHORITY This regulation is promulgated under the authority granted to the Needham Board of Health under Massachusetts General Laws Chapter 111, Section 31 which states that “boards of health may make reasonable health regulations”. SECTION 22.2 PURPOSE The Needham Board of Health has found that synthetic marijuana, consisting of plant or other material treated with various chemicals or other synthetic substances not approved for human consumption, may be marketed and sold as herbal incense in the greater Boston area, although they are being used in the same manner and for the same purposes as scheduled drugs. In addition, the use of these products has become particularly popular among teens and young adults. Based on information and reports from hospitals, emergency room doctors, and police agencies, individuals who use these products experience dangerous side effects including convulsions, hallucinations, and dangerously elevated heart rates. This is evidence that synthetic marijuana products are harmful if inhaled or consumed, and present a significant public health danger. These synthetic compounds and others have a high potential for abuse and lack of any accepted medical use, these dangerous products, while not approved for human consumption, are marketed and sold in a form that allows for such consumption, putting at risk the individuals who come into contact with them. Therefore, the Needham Board of Health adopts this regulation for the purpose and with the intent to protect the public health and safety of the Town of Needham and its residents from the threat posed by the availability and use of synthetic marijuana, synthetic stimulants, synthetic hallucinogens, and other dangerous products by prohibiting persons from trafficking in, possessing, and using them within the town. -

Hallucinogen-Like Actions of 5-Methoxy-N,N-Diisopropyltryptamine in Mice and Rats ⁎ W.E
Pharmacology, Biochemistry and Behavior 83 (2006) 122–129 www.elsevier.com/locate/pharmbiochembeh Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats ⁎ W.E. Fantegrossi a,b, , A.W. Harrington b, C.L. Kiessel b, J.R. Eckler c, R.A. Rabin c, J.C. Winter c, A. Coop d, K.C. Rice e, J.H. Woods b a Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, GA 30322, United States b Department of Pharmacology, University of Michigan Medical School, Ann Arbor, MI, United States c Department of Pharmacology and Toxicology, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY, United States d Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, MD, United States e Laboratory of Medicinal Chemistry, NIDDK, National Institutes of Health, Bethesda, MD Received 2 June 2005; received in revised form 7 December 2005; accepted 29 December 2005 Available online 3 February 2006 Abstract Few studies have examined the effects of 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT) in vivo. In these studies, 5-MeO-DIPT was tested in a drug-elicited head twitch assay in mice where it was compared to the structurally similar hallucinogen N,N-dimethyltryptamine (N,N- DMT) and challenged with the selective serotonin (5-HT)2A antagonist M100907, and in a lysergic acid diethylamide (LSD) discrimination assay in rats where its subjective effects were challenged with M100907 or the 5-HT1A selective antagonist WAY-100635. Finally, the affinity of 5- MeO-DIPT for three distinct 5-HT receptors was determined in rat brain. -

2 Spice English Presentation
Spice Spice contains no compensatory substances Специи не содержит компенсационные вещества Spice is a mix of herbs (shredded plant material) and manmade chemicals with mind-altering effects. It is often called “synthetic marijuana” because some of the chemicals in it are similar to ones in marijuana; but its effects are sometimes very different from marijuana, and frequently much stronger. It is most often labeled “Not for Human Consumption” and disguised as incense. Eliminationprocess • The synthetic agonists such as THC is fat soluble. • Probably, they are stored as THC in cell membranes. • Some of the chemicals in Spice, however, attach to those receptors more strongly than THC, which could lead to a much stronger and more unpredictable effect. • Additionally, there are many chemicals that remain unidentified in products sold as Spice and it is therefore not clear how they may affect the user. • Moreover, these chemicals are often being changed as the makers of Spice alter them to avoid the products being illegal. • To dissolve the Spice crystals Acetone is used endocannabinoids synhtetic THC cannabinoids CB1 and CB2 agonister Binds to cannabinoidreceptor CB1 CB2 - In the brain -in the immune system Decreased avtivity in the cell ____________________ Maria Ellgren Since some of the compounds have a longer toxic effects compared to naturally THC, as reported: • negative effects that often occur the day after consumption, as a general hangover , but without nausea, mentally slow, confused, distracted, impairment of long and short term memory • Other reports mention the qualitative impairment of cognitive processes and emotional functioning, like all the oxygen leaves the brain. -

Hb 185 an Act Listing Synthetic
62nd Legislature HB0185 AN ACT LISTING SYNTHETIC MARIJUANA AND SALVIA AS DANGEROUS DRUGS; REQUIRING RULEMAKING BY THE DEPARTMENT OF PUBLIC HEALTH AND HUMAN SERVICES; PROVIDING PENALTIES; AMENDING SECTIONS 44-12-102, 45-9-101, 45-9-102, 45-9-110, 50-31-306, 50-31-310, AND 50-32-222, MCA; AND PROVIDING AN IMMEDIATE EFFECTIVE DATE. BE IT ENACTED BY THE LEGISLATURE OF THE STATE OF MONTANA: Section 1. Section 44-12-102, MCA, is amended to read: "44-12-102. Things subject to forfeiture. (1) The following are subject to forfeiture: (a) all controlled substances that have been manufactured, distributed, prepared, cultivated, compounded, processed, or possessed in violation of Title 45, chapter 9; (b) all money, raw materials, products, and equipment of any kind that are used or intended for use in manufacturing, preparing, cultivating, compounding, processing, delivering, importing, or exporting any controlled substance in violation of Title 45, chapter 9, except items used or intended for use in connection with quantities of marijuana in amounts less than 60 grams; (c) except as provided in subsection (2)(d), all property that is used or intended for use as a container for anything enumerated in subsection (1)(a) or (1)(b); (d) except as provided in subsection (2), all conveyances, including aircraft, vehicles, and vessels, that are used or intended for use in any manner to facilitate the commission of a violation of Title 45, chapter 9; (e) all books, records, and research products and materials, including formulas, microfilm, tapes, and data, -
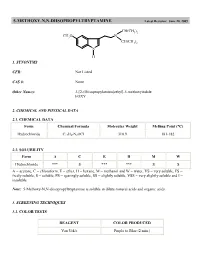
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

Introduced B.,Byhansen, 16
LB301 LB301 2021 2021 LEGISLATURE OF NEBRASKA ONE HUNDRED SEVENTH LEGISLATURE FIRST SESSION LEGISLATIVE BILL 301 Introduced by Hansen, B., 16. Read first time January 12, 2021 Committee: Judiciary 1 A BILL FOR AN ACT relating to the Uniform Controlled Substances Act; to 2 amend sections 28-401, 28-405, and 28-416, Revised Statutes 3 Cumulative Supplement, 2020; to redefine terms; to change drug 4 schedules and adopt federal drug provisions; to change a penalty 5 provision; and to repeal the original sections. 6 Be it enacted by the people of the State of Nebraska, -1- LB301 LB301 2021 2021 1 Section 1. Section 28-401, Revised Statutes Cumulative Supplement, 2 2020, is amended to read: 3 28-401 As used in the Uniform Controlled Substances Act, unless the 4 context otherwise requires: 5 (1) Administer means to directly apply a controlled substance by 6 injection, inhalation, ingestion, or any other means to the body of a 7 patient or research subject; 8 (2) Agent means an authorized person who acts on behalf of or at the 9 direction of another person but does not include a common or contract 10 carrier, public warehouse keeper, or employee of a carrier or warehouse 11 keeper; 12 (3) Administration means the Drug Enforcement Administration of the 13 United States Department of Justice; 14 (4) Controlled substance means a drug, biological, substance, or 15 immediate precursor in Schedules I through V of section 28-405. 16 Controlled substance does not include distilled spirits, wine, malt 17 beverages, tobacco, hemp, or any nonnarcotic substance if such substance 18 may, under the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved.