The Taxonomy of the Japanese Oak Red Scale Insect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Insect Color Vision: from Spectral Sensitivity to Visual Ecology
EN66CH23_vanderKooi ARjats.cls September 16, 2020 15:11 Annual Review of Entomology Evolution of Insect Color Vision: From Spectral Sensitivity to Visual Ecology Casper J. van der Kooi,1 Doekele G. Stavenga,1 Kentaro Arikawa,2 Gregor Belušic,ˇ 3 and Almut Kelber4 1Faculty of Science and Engineering, University of Groningen, 9700 Groningen, The Netherlands; email: [email protected] 2Department of Evolutionary Studies of Biosystems, SOKENDAI Graduate University for Advanced Studies, Kanagawa 240-0193, Japan 3Department of Biology, Biotechnical Faculty, University of Ljubljana, 1000 Ljubljana, Slovenia; email: [email protected] 4Lund Vision Group, Department of Biology, University of Lund, 22362 Lund, Sweden; email: [email protected] Annu. Rev. Entomol. 2021. 66:23.1–23.28 Keywords The Annual Review of Entomology is online at photoreceptor, compound eye, pigment, visual pigment, behavior, opsin, ento.annualreviews.org anatomy https://doi.org/10.1146/annurev-ento-061720- 071644 Abstract Annu. Rev. Entomol. 2021.66. Downloaded from www.annualreviews.org Copyright © 2021 by Annual Reviews. Color vision is widespread among insects but varies among species, depend- All rights reserved ing on the spectral sensitivities and interplay of the participating photore- Access provided by University of New South Wales on 09/26/20. For personal use only. ceptors. The spectral sensitivity of a photoreceptor is principally determined by the absorption spectrum of the expressed visual pigment, but it can be modified by various optical and electrophysiological factors. For example, screening and filtering pigments, rhabdom waveguide properties, retinal structure, and neural processing all influence the perceived color signal. -

Region of Nymphalidae and Libytheidae (Lepidoptera) from Japan
MORPHOLOGICAL STUDIES ON THE OCCIPITAL REGION OF NYMPHALIDAE AND LIBYTHEIDAE (LEPIDOPTERA) FROM JAPAN Takashi TSUBUKI and Nagao KOYAMA * .h2monji junior High School, 10-33, Kita6tsz{ha 1, Toshima-feu, Toleyo (170), 1opan ** Biological Laboratory, Shinshi2 Udeiversity, Cllada (386), 14pan CONTENTS I. Introduction・--・ny・・・・・・・・・・・・・・・・・-・-・・・・・・-・---・--t・-・---・ny・・・・-・-・・・-・-・・-・・・1 II. Materials and methods・-・・・・・・・・・t・・・・・・t・・・・・・・・・・・・・・`・・・・・・・・・・-・・・-・・・・・・-d・・・・・・・・・・・・2 III. General morphology of the occipital region ・・・・・・}・・・・・・・・・・・・・・・・・・・・・・・・・・・・・4 IV. Occipital structure of each species in Nymphaliclae and Libytheidae ・-ib-・・・・-・・・-・ny・・・・・--・-・・・・・・・-・・-・----・b-・・・----・-・・・-・・-・--・・-6 V. Phylogenical grouping of Nymphalidae based on the occipital structure・t・・・・・・・--・--・・-・-・・・・・・・・・・-・・・・-ny-・・・ny-・--・-----・H"""""".","".."."lg VI. Summary ・・・・-・-・-・・・・・・-・・----・・・-・・・・-・-・-・-----・・-------・-・----・23 VII. Literatures cited ・・・--・・・・・・・・・`・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・--・-・・-・・・・・・tt・・・・・・23 I. INTRODUCTION A considerable number of studies have been carried out on the occipital morphology of moths since YAGI and KoYAMA's report of 1963 (KoYAMA and MlyATA, 1969, 1970, 1975;MIYATA, 1971, 1972, 1973, 1974, 1975;MIyATA and KOYAMA, 1971, 1972, 1976). In the butterfiies, however, few papers were available concerning the occipital region, on which EHRucH (1958, a b), YAGI and KoyAMA (1963) and KoyAMA and OGAwA (1972) briefly described. Then, tried to study the occipital region of butterflies, the authors observed it preliminarily (TsuBuKI et aL 1975). The present paper deals with the occipital structure of Nymphalidae and Libytheidae from Japan with reference to its bearing on systematics. Before going further, the authors wish to express their gratitude to Prof. Dr. H. SAwADA and Prof. Dr. N. GOKAN, Tokyo University of Agriculture, for their advice and assistance. Thanks are also due to Mr. N. K6DA who gave valuable materials for this work, and to Mr. Y. YAGucm and the members of JEOL Ltd. -
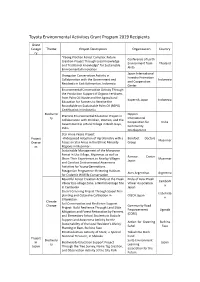
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

The Taxonomy of the Japanese Oak Red Scale Insect
Zootaxa 3630 (2): 291–307 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3630.2.5 http://zoobank.org/urn:lsid:zoobank.org:pub:373402BA-36A9-4323-AE9D-A84D89C47231 The taxonomy of the Japanese oak red scale insect, Kuwania quercus (Kuwana) (Hemiptera: Coccoidea: Kuwaniidae), with a generic diagnosis, a key to species and description of a new species from California SAN’AN WU1, NAN NAN1, PENNY GULLAN2 & JUN DENG1 1The Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University, Beijing, P. R. China 100083. E-mail: [email protected] 2Division of Evolution, Ecology and Genetics, Research School of Biology, The Australian National University, Canberra, A.C.T. 0200, Australia. E-mail: [email protected] Abstract The oak red scale insect, Kuwania quercus (Kuwana), was described from specimens collected from the bark of oak trees (Quercus species) in Japan. More recently, the species has been identified from California and China, but Californian specimens differ morphologically from Japanese material and are considered here to be a new species based on both morphological and molecular data. In this paper, an illustrated redescription of K. quercus is provided based on type specimens consisting of adult females, first-instar nymphs and intermediate-stage females, and a lectotype is designated for Sasakia quercus Kuwana. The new Californian species, Kuwania raygilli Wu & Gullan, is described and illustrated based on the adult female, first-instar nymph and intermediate-stage female. A new generic diagnosis for Kuwania Cockerell based on adult females and first-instar nymphs, and a key to species based on adult females are included. -

Mitochondrial Genomes of Hestina Persimilis and Hestinalis Nama (Lepidoptera, Nymphalidae): Genome Description and Phylogenetic Implications
insects Article Mitochondrial Genomes of Hestina persimilis and Hestinalis nama (Lepidoptera, Nymphalidae): Genome Description and Phylogenetic Implications Yupeng Wu 1,2,*, Hui Fang 1, Jiping Wen 2,3, Juping Wang 2, Tianwen Cao 2,* and Bo He 4 1 School of Environmental Science and Engineering, Taiyuan University of Science and Technology, Taiyuan 030024, China; [email protected] 2 College of Plant Protection, Shanxi Agricultural University, Taiyuan 030031, China; [email protected] (J.W.); [email protected] (J.W.) 3 Department of Horticulture, Taiyuan University, Taiyuan 030012, China 4 College of Life Sciences, Anhui Normal University, Wuhu 241000, China; [email protected] * Correspondence: [email protected] (Y.W.); [email protected] (T.C.) Simple Summary: In this study, the mitogenomes of Hestina persimilis and Hestinalis nama were obtained via sanger sequencing. Compared with other mitogenomes of Apaturinae butterflies, conclusions can be made that the mitogenomes of Hestina persimilis and Hestinalis nama are highly conservative. The phylogenetic trees build upon mitogenomic data showing that the relationships among Nymphalidae are similar to previous studies. Hestinalis nama is apart from Hestina, and closely related to Apatura, forming a monophyletic clade. Citation: Wu, Y.; Fang, H.; Wen, J.; Wang, J.; Cao, T.; He, B. Abstract: In this study, the complete mitochondrial genomes (mitogenomes) of Hestina persimilis Mitochondrial Genomes of Hestina and Hestinalis nama (Nymphalidae: Apaturinae) were acquired. The mitogenomes of H. persimilis persimilis and Hestinalis nama and H. nama are 15,252 bp and 15,208 bp in length, respectively. These two mitogenomes have the (Lepidoptera, Nymphalidae): typical composition, including 37 genes and a control region. -

Molecular Phylogeny and Taxonomy of Lepidoptera with Special Reference to Influence
Chapter 3 Molecularwith Special Phylogeny Reference and to InfluenceTaxonomy ofof Lepidoptera with Special Reference to Influence of Wolbachia Infection in the Genus Polytremis Weibin Jiang WeibinAdditional Jianginformation is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.69958 Abstract This chapter provides a case of genus Polytremis Mabille, 1904 (Lepidoptera: Hesperiidae), to explain the molecular phylogeny and taxonomy of Lepidoptera and the influence of Wolbachia infection. Earlier studies of Lepidoptera were focused mainly on the morpho- logical classification, population distribution, and identification of new species. As the supplementary to morphological research, analysis of DNA has been widely used in the phylogenetic studies of Lepidoptera. The study provides a conservative estimate that the Wolbachia infection rate in Polytremis nascens Leech (1893) is 31%, and no significant difference in the prevalence is found between the sexes. TheWolbachia infection mainly prevails in populations of P. nascens in southern China, which influence the diversity of mtDNA in P. nascens by a Wolbachia-induced sweep. The Wolbachia infection rate in Polytremis fukia Evans (1940) is 47% and shows a weak association existed between mito- chondrial DNA haplotypes and wFuk1 infection status. Keywords: Lepidoptera, microsatellite, mitochondrial genome, molecular phylogeny, taxonomy, Wolbachia 1. Introduction to molecular phylogeny and taxonomy of Lepidoptera Butterflies and mothsLepidoptera ( ) have long served as a model system for ecological and evo- lutionary studies on the basis of the high degree of diversity and complexity, which constitute one of the most diverse insect orders with more than 157,000 described species. -

Photonic Crystal Structure and Coloration of Wing Scales of Butterflies Exhibiting Selective Wavelength Iridescence
Materials 2012, 5, 754-771; doi:10.3390/ma5050754 OPEN ACCESS materials ISSN 1996-1944 www.mdpi.com/journal/materials Review Photonic Crystal Structure and Coloration of Wing Scales of Butterflies Exhibiting Selective Wavelength Iridescence Filip Mika 1,*, Jiřina Matějková-Plšková 1,†, Suratwadee Jiwajinda 2, Punyavee Dechkrong 2 and Makoto Shiojiri 3,‡ 1 Institute of Scientific Instruments of the ASCR, v.v.i., Královopolská 147, Brno 612 64, Czech Republic; E-Mail: [email protected] 2 Bioresources and Biodiversity Section, Central Laboratory and Greenhouse Complex, Kasetsart University, Kamphaengsaen Campus, Nakhonpathom 73140, Thailand; E-Mails: [email protected] (S.J.); [email protected] (P.D.) 3 Kyoto Institute of Technology, Kyoto 606-8585, Japan; E-Mail: [email protected] † Present address: Sadovského 14, Brno 61200, Czech Republic. ‡ Present address: 1-297 Wakiyama, Kyoto 618-0091, Japan. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +420-541-514-298; Fax: +420-541-514-402. Received: 21 March 2012; in revised form: 11 April 2012 / Accepted: 12 April 2012 / Published: 30 April 2012 Abstract: The coloration of butterflies that exhibit human visible iridescence from violet to green has been elucidated. Highly tilted multilayers of cuticle on the ridges, which were found in the scales of male S. charonda and E. mulciber butterflies, produce a limited-view, selective wavelength iridescence (ultraviolet (UV)~green) as a result of multiple interference between the cuticle-air layers. The iridescence from C. ataxus originates from multilayers in the groove plates between the ridges and ribs. The interference takes place between the top and bottom surfaces of each layer and incoherently between different layers. -

Lepidoptera) Communities in Korea: Conservation and Maintenance of Red Listed Species
Eur. J. Entomol. 112(4): 770–777, 2015 doi: 10.14411/eje.2015.099 ISSN 1210-5759 (print), 1802-8829 (online) Effect of military activity on butterfly (Lepidoptera) communities in Korea: Conservation and maintenance of red listed species SUNG-SOO KIM 1, TAE-SUNG KWON 2 and CHEOL MIN LEE 3, * 1 Research Institute for East Asian Environment and Biology, 293-27 Amsa 3 dong, Angdong-gu, Seoul 143-203, Republic of Korea; e-mail: [email protected] 2 Division of Forest Insect Pests and Diseases, Korea Forest Research Institute, 57 Hoegi-ro, Dongdaemun-gu, Seoul 130-712, Republic of Korea; e-mail: [email protected] 3 Center for Forest and Climate Change, Korea Forest Research Institute, 57 Hoegi-ro, Dongdaemun-gu, Seoul 130-712, Republic of Korea; e-mail: [email protected] Key words. Lepidoptera, butterflies, red listed species, military training area, grassland, conservation Abstract. Military training areas are increasingly recognized as areas of high biodiversity and habitats for many wild organisms, in- cluding threatened or endangered species. However, the information on the ecological value of military training areas is limited because it is difficult access these sites. The aim of this study is to evaluate the effect of military activity on butterfly communities. The survey was carried out in a military training area (MTA) at Inje-gun near the demilitarized zone (DMZ), Inje forest (IJF) a secondary forest and Gwangneung forest (GWF) an old growth forest, from April to October 2008 to 2011. IJF and GWF were selected in order to determine the characteristics of a butterfly community differed in a MTA. -

The Taxonomy of the Japanese Oak Red Scale Insect
Zootaxa 3630 (2): 291–307 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3630.2.5 http://zoobank.org/urn:lsid:zoobank.org:pub:373402BA-36A9-4323-AE9D-A84D89C47231 The taxonomy of the Japanese oak red scale insect, Kuwania quercus (Kuwana) (Hemiptera: Coccoidea: Kuwaniidae), with a generic diagnosis, a key to species and description of a new species from California SAN’AN WU1, NAN NAN1, PENNY GULLAN2 & JUN DENG1 1The Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University, Beijing, P. R. China 100083. E-mail: [email protected] 2Division of Evolution, Ecology and Genetics, Research School of Biology, The Australian National University, Canberra, A.C.T. 0200, Australia. E-mail: [email protected] Abstract The oak red scale insect, Kuwania quercus (Kuwana), was described from specimens collected from the bark of oak trees (Quercus species) in Japan. More recently, the species has been identified from California and China, but Californian specimens differ morphologically from Japanese material and are considered here to be a new species based on both morphological and molecular data. In this paper, an illustrated redescription of K. quercus is provided based on type specimens consisting of adult females, first-instar nymphs and intermediate-stage females, and a lectotype is designated for Sasakia quercus Kuwana. The new Californian species, Kuwania raygilli Wu & Gullan, is described and illustrated based on the adult female, first-instar nymph and intermediate-stage female. A new generic diagnosis for Kuwania Cockerell based on adult females and first-instar nymphs, and a key to species based on adult females are included. -
The Wildlife in Japan
T he 日本 Wildlife in の J apan 自然 The Wildlife in Japan Published in March 2015 Chuo-godochosha No. 5, 1-2-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-8975, Japan http://www.env.go.jp/ © Ministry of the Environment 2015 This brochure is printed on recycled paper. Edited and published by : Wildlife Division, Nature Conservation Bureau Editorial work : Japan Wildlife Research Center Design : artpost inc. Photos provided by : Hitoshi Imai, Harumi Iida, Kazuo Unno, Yoshiteru Eguchi, Katsumi Kawasaki, Kenji Kitaura, Masahide Kubota, Kano Koide, Yasumasa Kobayashi, Atsushi Sakurai, Yasushi Sugawara, Takao Sugeta, Hiroshi Takahashi, Tomonari Nakajima, Kenji Numata, Fumihiko Ban, Shinichi Hirasawa, Yukio Horiguchi, Misaki Mizukami, Kazuo Minato, Katsuhiko Mori, Noriaki Yamamoto, Shiro Yabe, Hisashi Yokota, Pika Fan Club and Society of Scientific Photography(SSP) 1 1 Flora of Japan The flora of Japan can be roughly classified into the following four categories based on the differences in temperature and precipitation: alpine zone, subalpine zone, summer-green broad-leaved forest zone and evergreen broad-leaved forest zone. The alpine zone is dominated by stone pines, the subalpine zone is dominated by spruces, and evergreen needle-leaved trees, the summer-green broad-leaved forest zone is dominated by deciduous broad-leaved trees such as Japanese beeches and Japanese oaks, and the evergreen broad-leaved forest zone is dominated by evergreen broad-leaved trees such as Yabutsubaki (Camellia japonica) and Shii (Castanopsis spp.) The Japanese archipelago is long, stretching from north to south, and has mountain ranges exceeding 3,000 m ; therefore, its vegetation changes both horizontally (with latitude) and vertically (with altitude). -
Lepidoptera. Robert Lucas
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Lepidoptera. Bearbeitet vou Dr. Robert Lucas in Reinickendorf. Al)l)ott, P. W. Rare Noctuae in the Isle of Wight. Entomologist 1896, p. 335. Arkle, J. (1). Apple-trees and wingless moths. Entomologist 1896, p. 193. Bezieht sich auf Mitchell's Artikel. — (2). Butterflies in the ehester District. Entomologist 1896, p. 195. — (3). Notes on the Season from the Chester District. t. c, p.215. Aurivillius, C. (1). Diagnosen neuer Lepidopteren aus dem Congo- Gebiete. OfversigtVet. Akad.Förhandlingar, 1896 p. 431 —^436. Behandelt werden: Mycalesis golo Aur. 9, Neptis lermanni n. sp., Euphaedra eberti n. S[)., Euryphene aurora n. sp., Cymothoe eris n. sp., Larinopoda hermansi n. sp. — (2). Beiträge zur Kenntnis der Insektenfauna von Kameran. 2. Tagfalter, 5. Farn. Hesperiidae. Entom. Tidskr. vol. XVII p. 279—292. Folgt der von Holland gegebenen Anordnung und behandelt No. 330—391 der Insektenfauna von Kamerun. Neu sind: Celaenor- rhinus intermixtus, Osmodes costatus, Ceratrichia fasciata und Caenides hidaroides. Sämmtliche 4 neue Arten sind abgebildet, ferner noch Caenides luehderi Plötz 9. Näher besprochen werden: Celaenorrhinus galenus Fabr., C. homeyeri Plötz, C. meditrina Hew., Osmudes adosus Mab., Ceratrichia flava, Andronymus leander Ploetz, Ortholexis melichroptera Karsch, Caenides luehderi Plötz, Rhopalo- campta forestan Cramer (Raupe) u. Rh. iphis Drury (Raupe). Zum Schluss eine Uebersicht über die Zahl der bis jetzt bekannten afrik. Tagfalter-Arten. Das kleine Gebiet zwischen dem Kamerun- Gebirge und dem N'Dian-Flusse: 392 Arten. — Das grosse Gebiet S.-Afrika's südl. vom Wendekreis des Steinbocks: 387 (nach Trimen). — Madagaskar: 255 (Mabille). — Sierra Leone: 211 (Schaus u. -

Variations in Butterfly Diversity in Relation to Habitat Type And
THE NOTTINGHAM TRENT UNIVERSITY BSc (Hons) BIOLOGICAL SCIENCES (ECOLOGY AND ENVIRONMENTAL MANAGEMENT) UNDERGRADUATE PROJECT REPORT TROPICAL BUTTERFLY DIVERSITIES WITHIN A BORNEAN RAINFOREST Lexias dirtea merguia in Kerangas habitat (Picture by Jessica Smallcombe) by SARAH NOLAN N0179587 THE NOTTINGHAM TRENT UNIVERSITY TROPICAL BUTTERFLY DIVERSITIES WITHIN A BORNEAN RAINFOREST by SARAH NOLAN Project submitted in partial fulfilment of the BSc (Honours) Biological Sciences Summer 2011 DECLARATION OF OWNERSHIP This submission is the result of my own work. All help and advice, other than that received from tutors, has been acknowledged and primary and secondary sources of information have been properly attributed. Should this statement prove to be untrue, I recognise the right and duty of the Board of Examiners to recommend what action should be taken in line with the University’s regulations on assessment contained in the Handbook. Signed: Date: Acknowledgements I would like to give massive thanks to Dr Chris Terrell – Nield for recommending and encouraging such a wonderful experience to me plus his commitment to my project throughout the whole year. To all of the Orangutan Tropical Peatland Project thank you for supporting my project and giving me the opportunity to carry out my own research. I must thank Jess Smallcombe for her supportive words, cuddles and strength to get me through the harder times. To Susan, Simon, Mark, Karen and Helen thank you for advising me through my project. Thank you to Lis and the Ibu’s for the food and my birthday cake, plus to Pac Bobby for collecting the ice and fresh food. Thank you to the Indonesian foresters who remained upbeat and worked hard for the whole project.