Region of Nymphalidae and Libytheidae (Lepidoptera) from Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Relationships and Historical Biogeography of Tribes and Genera in the Subfamily Nymphalinae (Lepidoptera: Nymphalidae)
Blackwell Science, LtdOxford, UKBIJBiological Journal of the Linnean Society 0024-4066The Linnean Society of London, 2005? 2005 862 227251 Original Article PHYLOGENY OF NYMPHALINAE N. WAHLBERG ET AL Biological Journal of the Linnean Society, 2005, 86, 227–251. With 5 figures . Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae) NIKLAS WAHLBERG1*, ANDREW V. Z. BROWER2 and SÖREN NYLIN1 1Department of Zoology, Stockholm University, S-106 91 Stockholm, Sweden 2Department of Zoology, Oregon State University, Corvallis, Oregon 97331–2907, USA Received 10 January 2004; accepted for publication 12 November 2004 We infer for the first time the phylogenetic relationships of genera and tribes in the ecologically and evolutionarily well-studied subfamily Nymphalinae using DNA sequence data from three genes: 1450 bp of cytochrome oxidase subunit I (COI) (in the mitochondrial genome), 1077 bp of elongation factor 1-alpha (EF1-a) and 400–403 bp of wing- less (both in the nuclear genome). We explore the influence of each gene region on the support given to each node of the most parsimonious tree derived from a combined analysis of all three genes using Partitioned Bremer Support. We also explore the influence of assuming equal weights for all characters in the combined analysis by investigating the stability of clades to different transition/transversion weighting schemes. We find many strongly supported and stable clades in the Nymphalinae. We are also able to identify ‘rogue’ -

Evolution of Insect Color Vision: from Spectral Sensitivity to Visual Ecology
EN66CH23_vanderKooi ARjats.cls September 16, 2020 15:11 Annual Review of Entomology Evolution of Insect Color Vision: From Spectral Sensitivity to Visual Ecology Casper J. van der Kooi,1 Doekele G. Stavenga,1 Kentaro Arikawa,2 Gregor Belušic,ˇ 3 and Almut Kelber4 1Faculty of Science and Engineering, University of Groningen, 9700 Groningen, The Netherlands; email: [email protected] 2Department of Evolutionary Studies of Biosystems, SOKENDAI Graduate University for Advanced Studies, Kanagawa 240-0193, Japan 3Department of Biology, Biotechnical Faculty, University of Ljubljana, 1000 Ljubljana, Slovenia; email: [email protected] 4Lund Vision Group, Department of Biology, University of Lund, 22362 Lund, Sweden; email: [email protected] Annu. Rev. Entomol. 2021. 66:23.1–23.28 Keywords The Annual Review of Entomology is online at photoreceptor, compound eye, pigment, visual pigment, behavior, opsin, ento.annualreviews.org anatomy https://doi.org/10.1146/annurev-ento-061720- 071644 Abstract Annu. Rev. Entomol. 2021.66. Downloaded from www.annualreviews.org Copyright © 2021 by Annual Reviews. Color vision is widespread among insects but varies among species, depend- All rights reserved ing on the spectral sensitivities and interplay of the participating photore- Access provided by University of New South Wales on 09/26/20. For personal use only. ceptors. The spectral sensitivity of a photoreceptor is principally determined by the absorption spectrum of the expressed visual pigment, but it can be modified by various optical and electrophysiological factors. For example, screening and filtering pigments, rhabdom waveguide properties, retinal structure, and neural processing all influence the perceived color signal. -
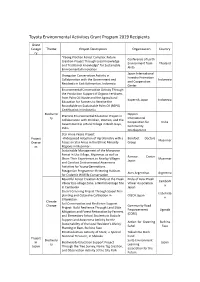
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

Northward Range Expansion of Southern Butterflies According to Climate Change in South Korea
Journal of Climate Change Research 2020, Vol. 11, No. 6-1, pp. 643~656 DOI: http://dx.doi.org/10.15531/KSCCR.2020.11.6.643 Northward Range Expansion of Southern Butterflies According to Climate Change in South Korea Adhikari, Pradeep* Jeon, Ja-Young** Kim, Hyun Woo*** Oh, Hong-Shik**** Adhikari, Prabhat***** and Seo, Changwan******† *Research Specialist, Environmental Impact Assessment Team, National Institute of Ecology, Korea **Researcher, Ecosystem Service Team, National Institute of Ecology, Korea / PhD student, Landscape Architecture, University of Seoul, Seoul, Korea ***Research Specialist, Eco Bank Team, National Institute of Ecology, Korea ****Professor, Interdisciplinary Graduate Program in Advanced Convergence Technology and Science and Faculty of Science Education, Jeju National University, South Korea *****Master student, Central Department of Botany, Tribhuvan University, Kathmandu, Nepal ******Chief Researcher, Division of Ecological Assessment, National Institute of Ecology, Korea ABSTRACT Climate change is one of the most influential factors on the range expansion of southern species into northern regions, which has been studied among insects, fish, birds and plants extensively in Europe and North America. However, in South Korea, few studies on the northward range expansion of insects, particularly butterflies, have been conducted. Therefore, we selected eight species of southern butterflies and calculated the potential species richness values and their range expansion in different provinces of Korea under two climate change scenarios (RCP 4.5 and RCP 8.5) using the maximum entropy (MaxEnt) modeling approach. Based on these model predictions, areas of suitable habitat, species richness, and species expansion of southern butterflies are expected to increase in provinces in the northern regions ( >36°N latitude), particularly in Chungcheongbuk, Gyeonggi, Gangwon, Incheon, and Seoul. -

The Taxonomy of the Japanese Oak Red Scale Insect
Zootaxa 3630 (2): 291–307 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3630.2.5 http://zoobank.org/urn:lsid:zoobank.org:pub:373402BA-36A9-4323-AE9D-A84D89C47231 The taxonomy of the Japanese oak red scale insect, Kuwania quercus (Kuwana) (Hemiptera: Coccoidea: Kuwaniidae), with a generic diagnosis, a key to species and description of a new species from California SAN’AN WU1, NAN NAN1, PENNY GULLAN2 & JUN DENG1 1The Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University, Beijing, P. R. China 100083. E-mail: [email protected] 2Division of Evolution, Ecology and Genetics, Research School of Biology, The Australian National University, Canberra, A.C.T. 0200, Australia. E-mail: [email protected] Abstract The oak red scale insect, Kuwania quercus (Kuwana), was described from specimens collected from the bark of oak trees (Quercus species) in Japan. More recently, the species has been identified from California and China, but Californian specimens differ morphologically from Japanese material and are considered here to be a new species based on both morphological and molecular data. In this paper, an illustrated redescription of K. quercus is provided based on type specimens consisting of adult females, first-instar nymphs and intermediate-stage females, and a lectotype is designated for Sasakia quercus Kuwana. The new Californian species, Kuwania raygilli Wu & Gullan, is described and illustrated based on the adult female, first-instar nymph and intermediate-stage female. A new generic diagnosis for Kuwania Cockerell based on adult females and first-instar nymphs, and a key to species based on adult females are included. -

Diversity Pattern of Butterfly Communities (Lepidoptera
International Scholarly Research Network ISRN Zoology Volume 2011, Article ID 818545, 8 pages doi:10.5402/2011/818545 Research Article DiversityPatternofButterflyCommunities (Lepidoptera, Papilionoidae) in Different Habitat Types in a Tropical Rain Forest of Southern Vietnam Lien Van Vu1 and Con Quang Vu2 1 Department of Biology, Vietnam National Museum of Nature, 18 Hoang Quoc Viet, Nghia Do, Cau Giay, Hanoi, Vietnam 2 Department of Insect Ecology, Institute of Ecology and Biological Resources, 18 Hoang Quoc Viet, Nghia Do, Cau Giay, Hanoi, Vietnam Correspondence should be addressed to Lien Van Vu, [email protected] Received 26 January 2011; Accepted 1 March 2011 Academic Editors: M. Griggio and V. Tilgar Copyright © 2011 L. V. Vu and C. Quang Vu. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Diversity of butterfly communities of a tropical rain forest of Bu Gia Map National Park in South Vietnam was studied in four different habitat types (the natural forest, the disturbed forest, the bamboo forest, and the stream sides in the forest) in December 2008 and April 2009. A total of 112 species with 1703 individuals of Papilionoidae (except Lycaenidae) were recorded. The proportion of rare species tends to decrease from the natural forest to the stream sides, while the proportion of common species tends to increase from the natural forest to the stream sides. The stream sides have the greatest individual number, while the disturbed forest contains the greatest species number. The bamboo forest has the least species and individual numbers. -

The Taxonomy of the Japanese Oak Red Scale Insect
Zootaxa 3630 (2): 291–307 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3630.2.5 http://zoobank.org/urn:lsid:zoobank.org:pub:373402BA-36A9-4323-AE9D-A84D89C47231 The taxonomy of the Japanese oak red scale insect, Kuwania quercus (Kuwana) (Hemiptera: Coccoidea: Kuwaniidae), with a generic diagnosis, a key to species and description of a new species from California SAN’AN WU1, NAN NAN1, PENNY GULLAN2 & JUN DENG1 1The Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University, Beijing, P. R. China 100083. E-mail: [email protected] 2Division of Evolution, Ecology and Genetics, Research School of Biology, The Australian National University, Canberra, A.C.T. 0200, Australia. E-mail: [email protected] Abstract The oak red scale insect, Kuwania quercus (Kuwana), was described from specimens collected from the bark of oak trees (Quercus species) in Japan. More recently, the species has been identified from California and China, but Californian specimens differ morphologically from Japanese material and are considered here to be a new species based on both morphological and molecular data. In this paper, an illustrated redescription of K. quercus is provided based on type specimens consisting of adult females, first-instar nymphs and intermediate-stage females, and a lectotype is designated for Sasakia quercus Kuwana. The new Californian species, Kuwania raygilli Wu & Gullan, is described and illustrated based on the adult female, first-instar nymph and intermediate-stage female. A new generic diagnosis for Kuwania Cockerell based on adult females and first-instar nymphs, and a key to species based on adult females are included. -

Ecology and Conservation Needs of Nymphalid Butterflies in Disturbed Tropical Forest of Eastern Himalayan Biodiversity Hotspot, Assam, India
International Journal of Biodiversity and Conservation Vol. 1(7) pp. 231-250, December, 2009 Available online http://www.academicjournals.org/ijbc ©2009 Academic Journals Full Length Research Paper Ecology and conservation needs of nymphalid butterflies in disturbed tropical forest of Eastern Himalayan biodiversity hotspot, Assam, India Malabika Kakati Saikia*, J. Kalita and P. K. Saikia Department of Zoology, Gauhati University, Gopinath Bardoloi Nagar, Jalukbari, Guwahati-781 014, Assam, India. Accepted 21 October, 2009 We examine the hypothesis, whether the diversity of Nymphalid butterflies in primary forest is related to vegetation structure and canopy openness and that this relationship differs between butterfly taxa in relation to phylogenetic differences in light and shade preferences. The study also examines whether the increasing diversity of butterflies in degraded tropical forest is associated with the loss of species with restricted geographical distribution. Present study has considered eight habitat parameters for habitat data collections and the t-test using equal variance, spearman rank correlation and multiple regressions were used for statistical analyses. Species diversity was analyzed using Margalef’s D indices that indicate both the species richness and abundance. Bootstrap method was used to compare the diversity among samples. PCA was carried out to examine the relationship between vegetation structure and species diversity in primary and degraded forest. The relationship between vegetation factor scores and species diversity at each sampling station in primary and degraded forest was analyzed using stepwise multiple regression. Results indicates that the butterflies species sampled in closed canopy forest had more restricted geographical distribution than those being sampled in disturbed forest. The species with greater light preference had significantly wider geographical distribution, whereas, the species with greater shade preferences had significantly narrower geographical distributions. -

Mitochondrial Genomes of Hestina Persimilis and Hestinalis Nama (Lepidoptera, Nymphalidae): Genome Description and Phylogenetic Implications
insects Article Mitochondrial Genomes of Hestina persimilis and Hestinalis nama (Lepidoptera, Nymphalidae): Genome Description and Phylogenetic Implications Yupeng Wu 1,2,*, Hui Fang 1, Jiping Wen 2,3, Juping Wang 2, Tianwen Cao 2,* and Bo He 4 1 School of Environmental Science and Engineering, Taiyuan University of Science and Technology, Taiyuan 030024, China; [email protected] 2 College of Plant Protection, Shanxi Agricultural University, Taiyuan 030031, China; [email protected] (J.W.); [email protected] (J.W.) 3 Department of Horticulture, Taiyuan University, Taiyuan 030012, China 4 College of Life Sciences, Anhui Normal University, Wuhu 241000, China; [email protected] * Correspondence: [email protected] (Y.W.); [email protected] (T.C.) Simple Summary: In this study, the mitogenomes of Hestina persimilis and Hestinalis nama were obtained via sanger sequencing. Compared with other mitogenomes of Apaturinae butterflies, conclusions can be made that the mitogenomes of Hestina persimilis and Hestinalis nama are highly conservative. The phylogenetic trees build upon mitogenomic data showing that the relationships among Nymphalidae are similar to previous studies. Hestinalis nama is apart from Hestina, and closely related to Apatura, forming a monophyletic clade. Citation: Wu, Y.; Fang, H.; Wen, J.; Wang, J.; Cao, T.; He, B. Abstract: In this study, the complete mitochondrial genomes (mitogenomes) of Hestina persimilis Mitochondrial Genomes of Hestina and Hestinalis nama (Nymphalidae: Apaturinae) were acquired. The mitogenomes of H. persimilis persimilis and Hestinalis nama and H. nama are 15,252 bp and 15,208 bp in length, respectively. These two mitogenomes have the (Lepidoptera, Nymphalidae): typical composition, including 37 genes and a control region. -

Occurrence Data of Southern Butterflies in South Korea
Data of Geology, Ecology, Oceanography, Space Science, Polar Science [Ecology] [Article] Occurrence data of southern butterflies in South Korea Hyun Woo Kim1, Youngho Cho2, Pradeep Adhikari3, Ja-Young Jeon3, Yong-gu Han4, Changwan Seo3* EcoBank Team, National Institute of Ecology1 Department of Research Policy, National Institute of Ecology2 Division of Ecological Assessment, National Institute of Ecology3 Team of National Ecosystem Survey, National Institute of Ecology4 (Received: 1 23 March 2020, revised: 22 April 2020, accepted: 29 April 2020) *Corresponding author: [email protected] Abstract: Background: In Northern hemisphere, climate change has shifted the habitats of many species including butterflies into the northern regions. Many researchers in Europe and North America have reported this type of northward shift of butterflies. Thus, we collected the species occurrence data of southern butterflies and presented in this study for understanding the impact of climate change on the southern butterflies in the future. New information: This study presents the 456 occurrence data of nine southern butterflies under five families in Korea. These data were selected from the 3rd National Ecosystem Survey (NES) conducted by National Institute of Environment Research (NIER) in Korea. Those will be a part of input data for MOTIVE-Ecosystem model, an integrative model to understand the influence of climate change and land cover change on the habitat suitability of sensitive, native and invasive species. These data will be important to the researchers and conservation agencies for understanding the current conditions of southern butterflies and developing conservation policy. Keywords: Climate change, southern butterflies, National Ecosystem Survey (NES), MOTIVE-Ecosystem model 1. -

Molecular Phylogeny and Taxonomy of Lepidoptera with Special Reference to Influence
Chapter 3 Molecularwith Special Phylogeny Reference and to InfluenceTaxonomy ofof Lepidoptera with Special Reference to Influence of Wolbachia Infection in the Genus Polytremis Weibin Jiang WeibinAdditional Jianginformation is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.69958 Abstract This chapter provides a case of genus Polytremis Mabille, 1904 (Lepidoptera: Hesperiidae), to explain the molecular phylogeny and taxonomy of Lepidoptera and the influence of Wolbachia infection. Earlier studies of Lepidoptera were focused mainly on the morpho- logical classification, population distribution, and identification of new species. As the supplementary to morphological research, analysis of DNA has been widely used in the phylogenetic studies of Lepidoptera. The study provides a conservative estimate that the Wolbachia infection rate in Polytremis nascens Leech (1893) is 31%, and no significant difference in the prevalence is found between the sexes. TheWolbachia infection mainly prevails in populations of P. nascens in southern China, which influence the diversity of mtDNA in P. nascens by a Wolbachia-induced sweep. The Wolbachia infection rate in Polytremis fukia Evans (1940) is 47% and shows a weak association existed between mito- chondrial DNA haplotypes and wFuk1 infection status. Keywords: Lepidoptera, microsatellite, mitochondrial genome, molecular phylogeny, taxonomy, Wolbachia 1. Introduction to molecular phylogeny and taxonomy of Lepidoptera Butterflies and mothsLepidoptera ( ) have long served as a model system for ecological and evo- lutionary studies on the basis of the high degree of diversity and complexity, which constitute one of the most diverse insect orders with more than 157,000 described species. -

ESPECIALLY for FIELD COLLECTORS (Under the Supervision of FRED T
1960 Journal of the Lepidopterists' Society 203 ESPECIALLY FOR FIELD COLLECTORS (Under the supervision of FRED T. THORNE, 1360 Merritt Dr., 1':1 Cajon, Calif., U.S.A.) BUTTERFLY HUNTING IN THE MOUNTAINS OF CENTRAL JAPAN by KAIYA KUBO The Tobira Spa is located between Mt. Hachibuse and the Utsukushi ga-hara Heights, and can be reached in an hour by bus from Matsumoto City, Nagano Prefecture, central Japan. In 1956, I stayed at one of the spa hotels from early June through the end of the summer season to collect and photograph butterflies there. In early summer when the fresh-green hills and valleys are adorned with scarlet mountain azaleas, the most abundant butterflies on the wing are perhaps Parnassius glacialis and Papilio maacki. The former is an elegant butterfly, though without red spots on the white wings, while the latter is one of the finest 'black' swallowtatils of Japan. These huge swallowtails are frequently seen gathering on the edges of way side pools and streams, or even the puddles on the bus road. The drinking butterflies were so numerous that I was tantalized over and over again at the sight of startled butterflies that scattered away from the road on which the bus was carrying me to the mountain resort. The number of butterlies getting together to take moisture varies from two to fifteen. They did not form an exclusive drinking society, for they were often jOined by other species e. g. Papilio machaon, P. bitInor, P. macilentus, Dichorragia nesimachus (Nymphalidre), Daimio tethys (Hesperiidre), etc. In photographing these large butterflies taking to water, I had no particular difficulties in approaching them, but every time I focussed my camera near the road I could not escape from inquisitive people, who only caused the butterflies to flyaway.