Dantchenko, A., Sourakov, A., and T. C. Emmel. 1996. Notes on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Populus Nigra Network
IN SITU CONSERVATION 79 In situ conservation Poplars and biodiversity PeterȱRotachȱ Department of Forest Sciences, Swiss Federal Institute of Technology, Zürich, Switzerland Floodplain forests, the natural habitat of indigenous black poplar (Populus nigra L.), are among the most diverse ecosystems in Europe (Gepp et al. 1985). In Austria, for example, it was estimated that at least 12 000 species of animals and plants regularly inhabit the floodplains of the Danube (Gepp et al. 1985). According to Gerken (1988) more than 1000 species of beetles, most of the indigenous amphibians, 400–500 species of large butterflies (more than one third of all existing species) and between 150 and 200 species of birds occur in different floodplain habitats. Table 1 shows the numbers of invertebrates that have been recorded in the floodplains of the Rhine. Table 1. Number of species of invertebrates in the floodplains of the Rhine, according to Tittizer and Krebs (1996) Order Number of species Mollusca (land snails) >60 Mollusca (water snails and mussels) 30–40 Odonata (dragonflies) 50 Coleoptera (beetles) >1000 Lepidoptera (butterflies) 1000 Arachnida (spiders) >100 Many of the species are highly specialized and depend on alluvial habitats. For example, 29% of the amphibians, 27% of the carabids, 20% of the reptiles and 12% of the dragonfly species in Switzerland occur uniquely or primarily in alluvial habitats (Walter et al. 1998). Undisturbed floodplain ecosystems are not only very rich in species, but also provide a unique or very important habitat for numerous threatened species and thus play a crucial role for species conservation. For insects, mammals, birds, reptiles and amphibians in Switzerland, for example, 17.5% of extinct species, 27% of those that are nearly extinct, 19% of highly threatened species and 11% of threatened species live exclusively, or primarily, in alluvial habitats (Walter et al. -

Evolution of Insect Color Vision: from Spectral Sensitivity to Visual Ecology
EN66CH23_vanderKooi ARjats.cls September 16, 2020 15:11 Annual Review of Entomology Evolution of Insect Color Vision: From Spectral Sensitivity to Visual Ecology Casper J. van der Kooi,1 Doekele G. Stavenga,1 Kentaro Arikawa,2 Gregor Belušic,ˇ 3 and Almut Kelber4 1Faculty of Science and Engineering, University of Groningen, 9700 Groningen, The Netherlands; email: [email protected] 2Department of Evolutionary Studies of Biosystems, SOKENDAI Graduate University for Advanced Studies, Kanagawa 240-0193, Japan 3Department of Biology, Biotechnical Faculty, University of Ljubljana, 1000 Ljubljana, Slovenia; email: [email protected] 4Lund Vision Group, Department of Biology, University of Lund, 22362 Lund, Sweden; email: [email protected] Annu. Rev. Entomol. 2021. 66:23.1–23.28 Keywords The Annual Review of Entomology is online at photoreceptor, compound eye, pigment, visual pigment, behavior, opsin, ento.annualreviews.org anatomy https://doi.org/10.1146/annurev-ento-061720- 071644 Abstract Annu. Rev. Entomol. 2021.66. Downloaded from www.annualreviews.org Copyright © 2021 by Annual Reviews. Color vision is widespread among insects but varies among species, depend- All rights reserved ing on the spectral sensitivities and interplay of the participating photore- Access provided by University of New South Wales on 09/26/20. For personal use only. ceptors. The spectral sensitivity of a photoreceptor is principally determined by the absorption spectrum of the expressed visual pigment, but it can be modified by various optical and electrophysiological factors. For example, screening and filtering pigments, rhabdom waveguide properties, retinal structure, and neural processing all influence the perceived color signal. -

Region of Nymphalidae and Libytheidae (Lepidoptera) from Japan
MORPHOLOGICAL STUDIES ON THE OCCIPITAL REGION OF NYMPHALIDAE AND LIBYTHEIDAE (LEPIDOPTERA) FROM JAPAN Takashi TSUBUKI and Nagao KOYAMA * .h2monji junior High School, 10-33, Kita6tsz{ha 1, Toshima-feu, Toleyo (170), 1opan ** Biological Laboratory, Shinshi2 Udeiversity, Cllada (386), 14pan CONTENTS I. Introduction・--・ny・・・・・・・・・・・・・・・・・-・-・・・・・・-・---・--t・-・---・ny・・・・-・-・・・-・-・・-・・・1 II. Materials and methods・-・・・・・・・・・t・・・・・・t・・・・・・・・・・・・・・`・・・・・・・・・・-・・・-・・・・・・-d・・・・・・・・・・・・2 III. General morphology of the occipital region ・・・・・・}・・・・・・・・・・・・・・・・・・・・・・・・・・・・・4 IV. Occipital structure of each species in Nymphaliclae and Libytheidae ・-ib-・・・・-・・・-・ny・・・・・--・-・・・・・・・-・・-・----・b-・・・----・-・・・-・・-・--・・-6 V. Phylogenical grouping of Nymphalidae based on the occipital structure・t・・・・・・・--・--・・-・-・・・・・・・・・・-・・・・-ny-・・・ny-・--・-----・H"""""".","".."."lg VI. Summary ・・・・-・-・-・・・・・・-・・----・・・-・・・・-・-・-・-----・・-------・-・----・23 VII. Literatures cited ・・・--・・・・・・・・・`・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・--・-・・-・・・・・・tt・・・・・・23 I. INTRODUCTION A considerable number of studies have been carried out on the occipital morphology of moths since YAGI and KoYAMA's report of 1963 (KoYAMA and MlyATA, 1969, 1970, 1975;MIYATA, 1971, 1972, 1973, 1974, 1975;MIyATA and KOYAMA, 1971, 1972, 1976). In the butterfiies, however, few papers were available concerning the occipital region, on which EHRucH (1958, a b), YAGI and KoyAMA (1963) and KoyAMA and OGAwA (1972) briefly described. Then, tried to study the occipital region of butterflies, the authors observed it preliminarily (TsuBuKI et aL 1975). The present paper deals with the occipital structure of Nymphalidae and Libytheidae from Japan with reference to its bearing on systematics. Before going further, the authors wish to express their gratitude to Prof. Dr. H. SAwADA and Prof. Dr. N. GOKAN, Tokyo University of Agriculture, for their advice and assistance. Thanks are also due to Mr. N. K6DA who gave valuable materials for this work, and to Mr. Y. YAGucm and the members of JEOL Ltd. -

Level Biology. Basic and Simplified Revision Notes
Systematic “A” level Biology. Basic and simplified Revision notes. SYSTEMATIC “A” LEVEL BIOLOGY. Basic and simplified revision notes. STANDARD TEACHING SYLABUS: 1. Cell biology or cytology………………………………………..………………………….2 Definition of cytology/cell biology, definition of the cell. Microscopy. light and electron microscopes their structure, mode of operation and comparison between them, microscope practical techniques. Cell theory, types of organism’s i.e prokaryotes and eukaryotes, comparison between Prokaryotes and eukaryotes, why cells are small? Structures of the cell, cell diversity.. Cell division. Types of cell division, events that occur during each type, comparison between them and the importance of each type. 2.Histology………………………………………………………………………………………..4 Definition, types of tissues, their structures and functions,adaptations of some tissues to suit their function. Levels of organization. ie unicellular level; tissue level; organ level; system level and organism level; advantages and disadvantages of being unicellular and multicelar organism; 3. Classification of living organisms…………………………………………………….65 Common terms used: classification, taxonomy, systematics, binomial nomenclature, dichotomous keys, taxonomic hierarchy, and five kingdom system: Animalia, Plantae, fungi, Protista and monera general characteristic of organismin each kingdom and the examples. 4. Transport of materials in living organisms………………………………………107 5. Chemicals of life………………………………………………………………………….150 DNA structure, RNA structure, DNA replication and protein -
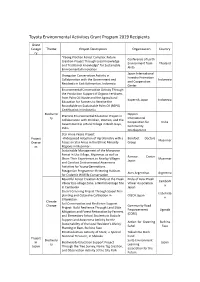
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

Issn 0972- 1800
ISSN 0972- 1800 VOLUME 22, NO. 2 QUARTERLY APRIL-JUNE, 2020 Date of Publication: 28th June, 2020 BIONOTES A Quarterly Newsletter for Research Notes and News On Any Aspect Related with Life Forms BIONOTES articles are abstracted/indexed/available in the Indian Science Abstracts, INSDOC; Zoological Record; Thomson Reuters (U.S.A); CAB International (U.K.); The Natural History Museum Library & Archives, London: Library Naturkundemuseum, Erfurt (Germany) etc. and online databases. Founder Editor Manuscripts Dr. R. K. Varshney, Aligarh, India Please E-mail to [email protected]. Board of Editors Guidelines for Authors Peter Smetacek, Bhimtal, India BIONOTES publishes short notes on any aspect of biology. Usually submissions are V.V. Ramamurthy, New Delhi, India reviewed by one or two reviewers. Jean Haxaire, Laplune, France Kindly submit a manuscript after studying the format used in this journal Vernon Antoine Brou, Jr., Abita Springs, (http://www.entosocindia.org/). Editor U.S.A. reserves the right to reject articles that do not Zdenek F. Fric, Ceske Budejovice, Czech adhere to our format. Please provide a contact Republic telephone number. Authors will be provided Stefan Naumann, Berlin, Germany with a pdf file of their publication. R.C. Kendrick, Hong Kong SAR Address for Correspondence Publication Policy Butterfly Research Centre, Bhimtal, Information, statements or findings Uttarakhand 263 136, India. Phone: +91 published are the views of its author/ source 8938896403. only. Email: [email protected] From Volume 21 Published by the Entomological Society of India (ESI), New Delhi (Nodal Officer: V.V. Ramamurthy, ESI, New Delhi) And Butterfly Research Centre, Bhimtal Executive Editor: Peter Smetacek Assistant Editor: Shristee Panthee Butterfly Research Trust, Bhimtal Published by Dr. -

Lepidoptera, Nymphalidae, Biblidinae) and Patterns of Morphological Similarity Among Species from Eight Tribes of Nymphalidae
Revista Brasileira de Entomologia http://dx.doi.org/10.1590/S0085-56262013005000006 External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae Luis Anderson Ribeiro Leite1,2, Mirna Martins Casagrande1,3 & Olaf Hermann Hendrik Mielke1,4 1Departamento de Zoologia, Setor de Ciências Biológicas, Universidade Federal do Paraná, Caixa Postal 19020, 81531–980 Curitiba-PR, Brasil. [email protected], [email protected], [email protected] ABSTRACT. External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae. The external structure of the integument of Dynamine postverta postverta (Cramer, 1779) is based on detailed morphological drawings and scanning electron microscopy. The data are compared with other species belonging to eight tribes of Nymphalidae, to assist future studies on the taxonomy and systematics of Neotropical Biblidinae. KEYWORDS. Abdomen; head; Insecta; morphology; Papilionoidea; thorax. Nymphalidae is a large cosmopolitan family of butter- served in dorsal view (Figs. 1–4). Two subspecies are recog- flies, with about 7,200 described species (Freitas & Brown nized according to Lamas (2004), Dynamine postverta Jr. 2004) and is perhaps the most well documented biologi- postverta (Cramer, 1779) distributed in South America and cally (Harvey 1991; Freitas & Brown Jr. 2004; Wahlberg et Dynamine postverta mexicana d’Almeida, 1952 with a dis- al. 2005). The systematic relationships are still somewhat tribution restricted to Central America. Several species sur- unclear with respect to its subfamilies, tribes and genera, and veys and other studies cite this species as Dynamine mylitta even after more than a century of studies on these groups, (DeVries 1987; Mielke 1994; Miller et al.1999; Freitas & these relationships still seem to confuse many who set out to Brown, Jr. -

Environmental Impact Assessment
Environmental Impact Assessment December 2013 IND: SASEC Road Connectivity Investment Program (formerly SASEC Road Connectivity Sector Project) Asian Highway 2 (India /Nepal Border to India/Bangladesh Border) Asian Highway 48 (India/Bhutan Border to India/Bangladesh Border) Prepared by Ministry of Roads Transport and Highways, Government of India and Public Works Department, Government of West Bengal for the Asian Development Bank. This is a revised version of the draft originally posted in July 2013 available on http://www.adb.org/projects/47341- 001/documents/. CURRENCY EQUIVALENTS (As of 30 April 2013) Currency unit – Indian rupee (INR) INR1.00 = $ 0.01818 $1.00 = INR 55.00 ABBREVIATION AADT Annual Average Daily Traffic AAQ Ambient air quality AAQM Ambient air quality monitoring ADB Asian Development Bank AH Asian Highway ASI Archaeological Survey of India BDL Below detectable limit BGL Below ground level BOD Biochemical oxygen demand BOQ Bill of quantity CCE Chief Controller of Explosives CGWA Central Ground Water Authority CITES Convention on International Trade in Endangered Species CO Carbon monoxide COD Chemical oxygen demand CPCB Central Pollution Control Board CSC Construction Supervision Consultant DFO Divisional Forest Officer DG Diesel generating set DO Dissolved oxygen DPR Detailed project report E&S Environment and social EA Executing agency EAC Expert Appraisal Committee EFP Environmental Focal Person EHS Environment Health and Safety EIA Environmental impact assessment EMOP Environmental monitoring plan EMP Environmental -

CBD First National Report
FIRST NATIONAL REPORT OF THE REPUBLIC OF SERBIA TO THE UNITED NATIONS CONVENTION ON BIOLOGICAL DIVERSITY July 2010 ACRONYMS AND ABBREVIATIONS .................................................................................... 3 1. EXECUTIVE SUMMARY ........................................................................................... 4 2. INTRODUCTION ....................................................................................................... 5 2.1 Geographic Profile .......................................................................................... 5 2.2 Climate Profile ...................................................................................................... 5 2.3 Population Profile ................................................................................................. 7 2.4 Economic Profile .................................................................................................. 7 3 THE BIODIVERSITY OF SERBIA .............................................................................. 8 3.1 Overview......................................................................................................... 8 3.2 Ecosystem and Habitat Diversity .................................................................... 8 3.3 Species Diversity ............................................................................................ 9 3.4 Genetic Diversity ............................................................................................. 9 3.5 Protected Areas .............................................................................................10 -

International Journal of Research Volume VIII, Issue VI, JUNE/2019
International Journal of Research ISSN NO:2236-6124 A Study on the Congregation of Adult Butterflies on Non-floral Resources at Different Locations in Jalpaiguri district of West Bengal, India Panchali Sengupta1*, Narayan Ghorai2 1Department of Zoology, West Bengal State University, Berunanpukaria, Malikapur, Barasat, District-24 Parganas (North), Kolkata-700126.West Bengal, India Email id: [email protected] 2Department of Zoology, West Bengal State University, Berunanpukaria, Malikapur, Barasat, District-24 Parganas (North), Kolkata-700126.West Bengal, India email id: [email protected] Abstract Several instances of puddling, as reported among different herbivore arthropods, appears quite interesting. Significantly, congregation of adult butterflies at several non-floral resources (wet soil/mud, animal dung, bird droppings, carrion, rotten/fermenting fruits) were examined at different locations in Jalpaiguri district adjacent to the tea estates, villages and agricultural tracts. Different species of papilionids and pierids congregate on wet soil patch and puddle collectively. However other species of nymphalid, lycaenid and hesperid are found to puddle individually, without associating with others on resources like excrements and carrion. Irrespective of any species newly emerged males, and aged females are found to puddle. Interestingly, each species belonging to a particular family have a specific range of puddling duration. Such specificity in puddling among species of a family could probably be associated with their need for a common nutrient. Keywords:, congregation, hesperid, lycaenid, nymphalid, papilionid, pierid *corresponding author Volume VIII, Issue VI, JUNE/2019 Page No:5877 International Journal of Research ISSN NO:2236-6124 Introduction Puddling is a widely recognised fascinating event in the life history of any herbivore arthropods except beetles targeted towards accumulation of specific micronutrient (Mollemann, 2010). -

Poleward Shifts in Geographical Ranges of Butterfly Species Associated with Regional Warming
letters to nature between 270 and 4,000 ms after target onset) and to ignore changes in the distractor. Failure to respond within a reaction-time window, responding to a change in the distractor or deviating the gaze (monitored with a scleral search Poleward shifts in coil) by more than 1Њ from the fixation point caused the trial to be aborted without reward. The change in the target and distractors was selected so as to geographical ranges of be challenging for the animal. In experiments 1 and 2 the animal correctly completed, on average, 79% of the trials, broke fixation in 11%, might have butterfly species associated responded to the distractor stimulus in 6% and responded too early or not at all in 5% of the trials. In Experiment 3 the corresponding values are 78, 13%, 8% with regional warming and 2%. In none of the three experiments was there a difference between the Camille Parmesan*†, Nils Ryrholm‡, Constantı´ Stefanescu§, performances for the two possible targets. Differences between average eye Jane K. Hillk, Chris D. Thomas¶, Henri Descimon#, positions during trials where one or the other stimulus was the target were Brian Huntleyk, Lauri Kaila!, Jaakko Kullberg!, very small, with only an average shift of 0.02Њ in the direction of the shift of Toomas Tammaru**, W. John Tennent††, position between the stimuli. Only correctly completed trials were considered. Jeremy A. Thomas‡‡ & Martin Warren§§ Firing rates were determined by computing the average neuronal response * National Center for Ecological Analysis and Synthesis, 735 State Street, across trials for 1,000 ms starting 200 ms after the beginning of the target Suite 300, Santa Barbara, California 93101, USA stimulus movement. -
Interspecific Variation in Competitor Avoidance and Foraging Success in Sap-Attracted Insects
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/270496969 Interspecific variation in competitor avoidance and foraging success in sap- attracted insects Article in European Journal of Entomology · November 2009 DOI: 10.14411/eje.2009.066 CITATIONS READS 0 10 1 author: Jiichiro Yoshimoto University of the Valley of Guatemala 12 PUBLICATIONS 58 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Climate change effects on the biodiversity of the seasonally dry tropical forests of Motagua Valley in Guatemala View project All content following this page was uploaded by Jiichiro Yoshimoto on 28 January 2019. The user has requested enhancement of the downloaded file. Eur. J. Entomol. 106: 529–533, 2009 http://www.eje.cz/scripts/viewabstract.php?abstract=1484 ISSN 1210-5759 (print), 1802-8829 (online) Interspecific variation in competitor avoidance and foraging success in sap-attracted insects JIICHIRO YOSHIMOTO* Laboratory of Insect Ecology, Graduate School of Agriculture, Kyoto University, Kitashirakawa Oiwake-cho, Sakyo-ku, Kyoto 606-8502, Japan Key words. Aggressive interactions, community, foraging strategy, interference competition, resources, tree sap Abstract. Many insect species attracted to fermenting sap often fight for access to this resource, which results in the establishment of interspecific dominance hierarchies. In one such system, the hornet Vespa mandarinia (Hymenoptera: Vespidae) behaviourally dominates during the daytime and several subordinate species avoid aggressive interactions in various ways. In order to elucidate the interspecific variation in competitor-avoidance behaviour and its subsequent effect on foraging success, the behaviour of species of hornets, beetles and butterflies at patches (exudation spots) in Japan was recorded.