The Taxonomic Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Celtis Tenuifolia) in Ontario
Photo: Allen Woodliffe Dwarf Hackberry (Celtis tenuifolia) in Ontario Ontario Recovery Strategy Series Recovery strategy prepared under the Endangered Species Act, 2007 2013 Ministry of Natural Resources About the Ontario Recovery Strategy Series This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada. What is recovery? What’s next? Recovery of species at risk is the process by which the Nine months after the completion of a recovery strategy decline of an endangered, threatened, or extirpated a government response statement will be published species is arrested or reversed, and threats are which summarizes the actions that the Government of removed or reduced to improve the likelihood of a Ontario intends to take in response to the strategy. species’ persistence in the wild. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and What is a recovery strategy? conservationists. Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to For more information achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the To learn more about species at risk recovery in Ontario, survival and recovery of the species. It also makes please visit the Ministry of Natural Resources Species at recommendations on the objectives for protection and Risk webpage at: www.ontario.ca/speciesatrisk recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. -

Caterpillars Moths Butterflies Woodies
NATIVE Caterpillars Moths and utter flies Band host NATIVE Hackberry Emperor oodies PHOTO : Megan McCarty W Double-toothed Prominent Honey locust Moth caterpillar Hackberry Emperor larva PHOTO : Douglas Tallamy Big Poplar Sphinx Number of species of Caterpillars n a study published in 2009, Dr. Oaks (Quercus) 557 Beeches (Fagus) 127 Honey-locusts (Gleditsia) 46 Magnolias (Magnolia) 21 Double-toothed Prominent ( Nerice IDouglas W. Tallamy, Ph.D, chair of the Cherries (Prunus) 456 Serviceberry (Amelanchier) 124 New Jersey Tea (Ceanothus) 45 Buttonbush (Cephalanthus) 19 bidentata ) larvae feed exclusively on elms Department of Entomology and Wildlife Willows (Salix) 455 Larches or Tamaracks (Larix) 121 Sycamores (Platanus) 45 Redbuds (Cercis) 19 (Ulmus), and can be found June through Ecology at the University of Delaware Birches (Betula) 411 Dogwoods (Cornus) 118 Huckleberry (Gaylussacia) 44 Green-briar (Smilax) 19 October. Their body shape mimics the specifically addressed the usefulness of Poplars (Populus) 367 Firs (Abies) 117 Hackberry (Celtis) 43 Wisterias (Wisteria) 19 toothed shape of American elm, making native woodies as host plants for our Crabapples (Malus) 308 Bayberries (Myrica) 108 Junipers (Juniperus) 42 Redbay (native) (Persea) 18 them hard to spot. The adult moth is native caterpillars (and obviously Maples (Acer) 297 Viburnums (Viburnum) 104 Elders (Sambucus) 42 Bearberry (Arctostaphylos) 17 small with a wingspan of 3-4 cm. therefore moths and butterflies). Blueberries (Vaccinium) 294 Currants (Ribes) 99 Ninebark (Physocarpus) 41 Bald cypresses (Taxodium) 16 We present here a partial list, and the Alders (Alnus) 255 Hop Hornbeam (Ostrya) 94 Lilacs (Syringa) 40 Leatherleaf (Chamaedaphne) 15 Honey locust caterpillar feeds on honey number of Lepidopteran species that rely Hickories (Carya) 235 Hemlocks (Tsuga) 92 Hollies (Ilex) 39 Poison Ivy (Toxicodendron) 15 locust, and Kentucky coffee trees. -

Lepidoptera, Nymphalidae, Biblidinae) and Patterns of Morphological Similarity Among Species from Eight Tribes of Nymphalidae
Revista Brasileira de Entomologia http://dx.doi.org/10.1590/S0085-56262013005000006 External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae Luis Anderson Ribeiro Leite1,2, Mirna Martins Casagrande1,3 & Olaf Hermann Hendrik Mielke1,4 1Departamento de Zoologia, Setor de Ciências Biológicas, Universidade Federal do Paraná, Caixa Postal 19020, 81531–980 Curitiba-PR, Brasil. [email protected], [email protected], [email protected] ABSTRACT. External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae. The external structure of the integument of Dynamine postverta postverta (Cramer, 1779) is based on detailed morphological drawings and scanning electron microscopy. The data are compared with other species belonging to eight tribes of Nymphalidae, to assist future studies on the taxonomy and systematics of Neotropical Biblidinae. KEYWORDS. Abdomen; head; Insecta; morphology; Papilionoidea; thorax. Nymphalidae is a large cosmopolitan family of butter- served in dorsal view (Figs. 1–4). Two subspecies are recog- flies, with about 7,200 described species (Freitas & Brown nized according to Lamas (2004), Dynamine postverta Jr. 2004) and is perhaps the most well documented biologi- postverta (Cramer, 1779) distributed in South America and cally (Harvey 1991; Freitas & Brown Jr. 2004; Wahlberg et Dynamine postverta mexicana d’Almeida, 1952 with a dis- al. 2005). The systematic relationships are still somewhat tribution restricted to Central America. Several species sur- unclear with respect to its subfamilies, tribes and genera, and veys and other studies cite this species as Dynamine mylitta even after more than a century of studies on these groups, (DeVries 1987; Mielke 1994; Miller et al.1999; Freitas & these relationships still seem to confuse many who set out to Brown, Jr. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

Insects That Feed on Trees and Shrubs
INSECTS THAT FEED ON COLORADO TREES AND SHRUBS1 Whitney Cranshaw David Leatherman Boris Kondratieff Bulletin 506A TABLE OF CONTENTS DEFOLIATORS .................................................... 8 Leaf Feeding Caterpillars .............................................. 8 Cecropia Moth ................................................ 8 Polyphemus Moth ............................................. 9 Nevada Buck Moth ............................................. 9 Pandora Moth ............................................... 10 Io Moth .................................................... 10 Fall Webworm ............................................... 11 Tiger Moth ................................................. 12 American Dagger Moth ......................................... 13 Redhumped Caterpillar ......................................... 13 Achemon Sphinx ............................................. 14 Table 1. Common sphinx moths of Colorado .......................... 14 Douglas-fir Tussock Moth ....................................... 15 1. Whitney Cranshaw, Colorado State University Cooperative Extension etnomologist and associate professor, entomology; David Leatherman, entomologist, Colorado State Forest Service; Boris Kondratieff, associate professor, entomology. 8/93. ©Colorado State University Cooperative Extension. 1994. For more information, contact your county Cooperative Extension office. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, -

Sugarberry Plant Fact Sheet
Plant Fact Sheet Wildlife: Many species of songbirds including SUGARBERRY mockingbirds and robins eat the fruit and use the tree for nesting habitat. It is a larval and nectar host for two Celtis laevigata Willd. butterflies: hackberry emperor (Asterocampa celtis) and Plant Symbol = CELA American snout (Libytheana carineta). White-tailed deer browse the leaves and fruit. Contributed by: USDA NRCS Plant Materials Program Other Uses: Sugarberry is used for furniture, athletic goods, firewood, and plywood. It has limited use for flooring, creating, and for wood posts. It is used as an ornamental and as a street tree in residential areas in the lower South Status Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g. threatened or endangered species, state noxious status, and wetland indicator values). Description and Adaptation Sugarberry is a native tree that can grow up to 80 feet in height and up to 3 feet in diameter. It is a short lived tree, probably living not more than 150 years. It has a broad crown formed by spreading branches that are often drooped. The bark is light gray in color and can be smooth or covered with corky warts. The branchlets are covered with short hairs at first and eventually they become smooth. The leaves are alternated, simple, and slightly serrate. The leaves are 2-4 inches long and 1 to 2 Robert H. Mohlenbrock. USDA SCS, 1989. Midwest wetland flora: inches wide. The lance-shaped leaves gradually taper to a Field office illustrated guide to plant species. -
Common Species
Common Species on District Lands The Southwest Florida Water Management District (District) manages water resources in the west- central portion of Florida according to legislative mandates. The District uses public land acquisition funds to protect key lands for water resources. The natural communities, plants and animals on these lands also benefi t from a level of protection that can be experienced and enjoyed through recreational opportunities made available on District lands. Every year, approximately 2.5 million people visit the more than 436,000 acres of public conservation lands acquired by the District and its partners. The lands are open to the public for activities such as hiking, bicycling, hunting, horseback riding, fi shing, camping, picnicking and studying nature. The District provides these recreational opportunities so that residents may experience healthy natural communities. An array of natural landscapes is available from the meandering Outstanding Florida Southwest Florida Water Management District Waters riverine systems, the cypress domes and strands, swamps and hammocks, to the scrub, sandhill, and pine fl atwoods. The Common Species on District Lands brochure provides insight to what you may experience while recreating on public lands managed by the District. CONSERVATION LANDS Southwest Florida Water Management District Mammals Southwest Florida Water Management District iStock W Barbour - Smithsonian Institute Roger Virginia Brazilian Opossum Free-Tailed Bat Didelphis virginiana Tadarida brasiliensis iStock Ivy -
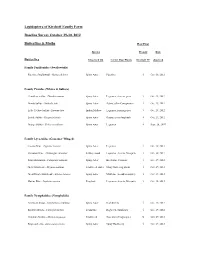
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Conservation of the Arogos Skipper, Atrytone Arogos Arogos (Lepidoptera: Hesperiidae) in Florida Marc C
Conservation of the Arogos Skipper, Atrytone arogos arogos (Lepidoptera: Hesperiidae) in Florida Marc C. Minno St. Johns River Water Management District P.O. Box 1429, Palatka, FL 32177 [email protected] Maria Minno Eco-Cognizant, Inc., 600 NW 35th Terrace, Gainesville, FL 32607 [email protected] ABSTRACT The Arogos skipper is a rare and declining butterfly found in native grassland habitats in the eastern and mid- western United States. Five distinct populations of the butterfly occur in specific parts of the range. Atrytone arogos arogos once occurred from southern South Carolina through eastern Georgia and peninsular Florida as far south as Miami. This butterfly is currently thought to be extirpated from South Carolina and Georgia. The six known sites in Florida for A. arogos arogos are public lands with dry prairie or longleaf pine savanna having an abundance of the larval host grass, Sorghastrum secundum. Colonies of the butterfly are threat- ened by catastrophic events such as wild fires, land management activities or no management, and the loss of genetic integrity. The dry prairie preserves of central Florida will be especially important to the recovery of the butterfly, since these are some of the largest and last remaining grasslands in the state. It may be possible to create new colonies of the Arogos skipper by releasing wild-caught females or captive-bred individuals into currently unoccupied areas of high quality habitat. INTRODUCTION tered colonies were found in New Jersey, North Carolina, South Carolina, Florida, and Mississippi. The three re- gions where the butterfly was most abundant included The Arogos skipper (Atrytone arogos) is a very locally the New Jersey pine barrens, peninsular Florida, and distributed butterfly that occurs only in the eastern and southeastern Mississippi. -

BUTTERFLIES in Thewest Indies of the Caribbean
PO Box 9021, Wilmington, DE 19809, USA E-mail: [email protected]@focusonnature.com Phone: Toll-free in USA 1-888-721-3555 oror 302/529-1876302/529-1876 BUTTERFLIES and MOTHS in the West Indies of the Caribbean in Antigua and Barbuda the Bahamas Barbados the Cayman Islands Cuba Dominica the Dominican Republic Guadeloupe Jamaica Montserrat Puerto Rico Saint Lucia Saint Vincent the Virgin Islands and the ABC islands of Aruba, Bonaire, and Curacao Butterflies in the Caribbean exclusively in Trinidad & Tobago are not in this list. Focus On Nature Tours in the Caribbean have been in: January, February, March, April, May, July, and December. Upper right photo: a HISPANIOLAN KING, Anetia jaegeri, photographed during the FONT tour in the Dominican Republic in February 2012. The genus is nearly entirely in West Indian islands, the species is nearly restricted to Hispaniola. This list of Butterflies of the West Indies compiled by Armas Hill Among the butterfly groupings in this list, links to: Swallowtails: family PAPILIONIDAE with the genera: Battus, Papilio, Parides Whites, Yellows, Sulphurs: family PIERIDAE Mimic-whites: subfamily DISMORPHIINAE with the genus: Dismorphia Subfamily PIERINAE withwith thethe genera:genera: Ascia,Ascia, Ganyra,Ganyra, Glutophrissa,Glutophrissa, MeleteMelete Subfamily COLIADINAE with the genera: Abaeis, Anteos, Aphrissa, Eurema, Kricogonia, Nathalis, Phoebis, Pyrisitia, Zerene Gossamer Wings: family LYCAENIDAE Hairstreaks: subfamily THECLINAE with the genera: Allosmaitia, Calycopis, Chlorostrymon, Cyanophrys, -

The Lesser Purple Emperor, Apatura Ilia: from Mimesis to Biomimetics
Faraday Discussions The Lesser Purple Emperor, Apatura ilia: from mimesis to biomimetics Journal: Faraday Discussions Manuscript ID FD-ART-03-2020-000036.R1 Article Type: Paper Date Submitted by the 27-Mar-2020 Author: Complete List of Authors: Schenk, Franziska; Birmingham City University, School of Art Stavenga, Doekele; University Groningen, Computational Physics Page 1 of 18 Faraday Discussions 1 The Lesser Purple Emperor butterfly, Apatura ilia: from mimesis to biomimetics 2 3 Franziska Schenk1,* and Doekele G. Stavenga2 4 1 School of Art, Birmingham Institute of Art and Design, Birmingham City University, 5 Birmingham, U.K. 6 2 Zernike Institute for Advanced Materials, University of Groningen, Groningen, the 7 Netherlands 8 * E-Mail: [email protected] 9 10 Abstract. Until now, hues as dynamic as those adorning the Apatura Emperor butterflies have 11 never been encountered in the painting world. Unlike and unmatched by the chemical pigments 12 traditionally found on the painter’s palette, the Emperor’s wings are studded with strongly 13 reflecting iridescent scales that are structured like those of the iconic morpho butterflies. The 14 scale ridges act as diffractive multilayers, giving rise to narrow-band reflectance spectra. All 15 scales together create a vividly purple iridescent wing colouration that is observed within a 16 narrow angular range only. Recently, synthetic structures analogous to the multilayer reflectors 17 found on butterfly wings have been developed, referred to as effect pigments. Artists can obtain 18 vital clues on how to adapt and adopt these challenging new materials for painting, by tracing the 19 origin of biomimetics back to the ancient concept of mimesis and building on the knowledge 20 accumulated by optical studies. -

Butterflies and Moths of Dominican Republic
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail