Environmental Applications of Small Unmanned Aircraft
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Threat Agents Call Poison Control 24/7 for Treatment Information 1.800.222.1222 Blood Nerve Blister Pulmonary Metals Toxins
CHEMICAL THREAT AGENTS CALL POISON CONTROL 24/7 FOR TREATMENT INFORMATION 1.800.222.1222 BLOOD NERVE BLISTER PULMONARY METALS TOXINS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS • Vertigo • Diarrhea, diaphoresis • Itching • Upper respiratory tract • Cough • Shock • Tachycardia • Urination • Erythema irritation • Metallic taste • Organ failure • Tachypnea • Miosis • Yellowish blisters • Rhinitis • CNS effects • Cyanosis • Bradycardia, bronchospasm • Flu-like symptoms • Coughing • Shortness of breath • Flu-like symptoms • Emesis • Delayed eye irritation • Choking • Flu-like symptoms • Nonspecific neurological • Lacrimation • Delayed pulmonary edema • Visual disturbances symptoms • Salivation, sweating INDICATIVE LAB TESTS INDICATIVE LAB TESTS INDICATIVE LAB TEST INDICATIVE LAB TESTS INDICATIVE LAB TESTS INDICATIVE LAB TESTS • Increased anion gap • Decreased cholinesterase • Thiodiglycol present in urine • Decreased pO2 • Proteinuria None Available • Metabolic acidosis • Increased anion gap • Decreased pCO2 • Renal assessment • Narrow pO2 difference • Metabolic acidosis • Arterial blood gas between arterial and venous • Chest radiography samples DEFINITIVE TEST DEFINITIVE TEST DEFINITIVE TEST DEFINITIVE TESTS DEFINITIVE TESTS • Blood cyanide levels • Urine nerve agent • Urine blister agent No definitive tests available • Blood metals panel • Urine ricinine metabolites metabolites • Urine metals panel • Urine abrine POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS • Hydrogen Cyanide -

Regulated Substance List
INSTRUCTIONS FOR THE UNIFIED PROGRAM (UP) FORM REGULATED SUBSTANCE LIST CHEMICAL NAME CAS # TQ Listing CHEMICAL NAME CAS # TQ Listing (Lbs) Basis (Lbs) Basis Acetaldehyde 75-07-0 10,000 g Cantharidin 56-25-7 100/10,0001 * Acetone Cyanohydrin 75-86-5 1,000 Carbachol Chloride 51-83-2 500/10,0001 Acetone Thiosemicarbazide 1752-30-3 1,000/10,0001 Acetylene (Ethyne) 74-86-2 10,000 f Carbamic Acid, Methyl-,o- Acrolein (2-Propenal) 107-02-8 500 b (((2,4-Dimethyl-1,3-Dithiolan- Acrylamide 79-06-1 1,000/10,0001 2-YL) Methylene)Amino)- 26419-73-8 100/10,0001 Acrylonitrile (2- Propenenitrile) 107-13-1 10,000 b Carbofuran 1563-66-2 10/10,0001 Acrylyl Chloride Carbon Disulfide 75-15-0 10,000 b (2-Propenoyl Chloride) 814-68-6 100 b Carbon Oxysulfide Aldicarb 116-06-3 100/10,0001 (Carbon Oxide Sulfide (COS)) 463-58-1 10,000 f Aldrin 309-00-2 500/10,0001 Chlorine 7782-50-5 100 a,b Allyl Alcohol (2-Propen-1-ol) 107-18-6 1,000 b Chlorine Dioxide Allylamine (2-Propen-1-Amine) 107-11-9 500 b (Chlorine Oxide (ClO2)) 10049-04-4 1,000 c Aluminum Phosphide 20859-73-8 500 Chlorine Monoxide (Chlorine Oxide) 7791-21-1 10,000 f Aminopterin 54-62-6 500/10,0001 Chlormequat Chloride 999-81-5 100/10,0001 Amiton Oxalate 3734-97-2 100/10,0001 Chloroacetic Acid 79-11-8 100/10,0001 Ammonia, Anhydrous 2 7664-41-7 500 a,b Chloroform 67-66-3 10,000 b Ammonia, Aqueous Chloromethyl Ether (conc 20% or greater) 7664-41-7 20,000 a,b (Methane,Oxybis(chloro-) 542-88-1 100 b * Aniline 62-53-3 1,000 Chloromethyl Methyl Ether Antimycin A 1397-94-0 1,000/10,0001 (Chloromethoxymethane) -

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

Warfare Agents for Modeling Airborne Dispersion in and Around Buildings
LBNL-45475 ERNEST ORLANDO LAWRENCE BERKELEY NATIn NAL LABORATORY Databaseof Physical,Chemicaland ToxicologicalPropertiesof Chemical and Biological(CB)WarfitreAgentsfor ModelingAirborneDispersionIn and AroundBuildings TracyThatcher,RichSextro,andDonErmak Environmental Energy Technologies Division DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor The Regents of the University of Catifomia, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of anY information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommend at i on, or favoring by the United States Government or any agency thereof, or The Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof, or The Regents of the University of California. Ernest Orlando Lawrence Berkeley National Laboratory is an equal opportunity employer. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced -

Chemical Warfare Agent (CWA) Identification Overview
Physicians for Human Rights Chemical Warfare Agent (CWA) Identification Overview Chemical Warfare Agent Identification Fact Sheet Series Table of Contents This Chemical Warfare Agent (CWA) Identification Fact Sheet is part 2 Physical Properties of a Physicians for Human Rights (PHR) series designed to fill a gap in 2 VX (Nerve Agent) 2 Sarin (Nerve Agent) knowledge among medical first responders to possible CWA attacks. 2 Tabun (Nerve Agent) This document in particular outlines differences between a select 2 BZ (Incapacitating Agent) group of vesicants and nerve agents, the deployment of which would 2 Mustard Gas (Vesicant) necessitate emergency medical treatment and documentation. 3 Collecting Samples to Test for Exposure 4 Protection PHR hopes that, by referencing these fact sheets, medical professionals 5 Symptoms may be able to correctly diagnose, treat, and document evidence of 6 Differential Diagnosis exposure to CWAs. Information in this fact sheet has been compiled from 8 Decontimanation 9 Treatment publicly available sources. 9 Abbreviations A series of detailed CWA fact sheets outlining in detail those properties and treatment regimes unique to each CWA is available at physiciansforhumanrights.org/training/chemical-weapons. phr.org Chemical Warfare Agent (CWA) Identification Overview 1 Collect urine samples, and blood and hair samples if possible, immediately after exposure Physical Properties VX • A lethal dose (10 mg) of VX, absorbed through the skin, can kill within minutes (Nerve Agent) • Can remain in environment for weeks -
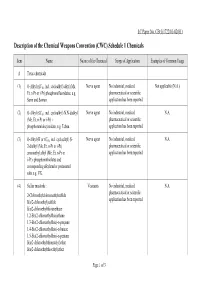
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

Nerve Agent - Lntellipedia Page 1 Of9 Doc ID : 6637155 (U) Nerve Agent
This document is made available through the declassification efforts and research of John Greenewald, Jr., creator of: The Black Vault The Black Vault is the largest online Freedom of Information Act (FOIA) document clearinghouse in the world. The research efforts here are responsible for the declassification of MILLIONS of pages released by the U.S. Government & Military. Discover the Truth at: http://www.theblackvault.com Nerve Agent - lntellipedia Page 1 of9 Doc ID : 6637155 (U) Nerve Agent UNCLASSIFIED From lntellipedia Nerve Agents (also known as nerve gases, though these chemicals are liquid at room temperature) are a class of phosphorus-containing organic chemicals (organophosphates) that disrupt the mechanism by which nerves transfer messages to organs. The disruption is caused by blocking acetylcholinesterase, an enzyme that normally relaxes the activity of acetylcholine, a neurotransmitter. ...--------- --- -·---- - --- -·-- --- --- Contents • 1 Overview • 2 Biological Effects • 2.1 Mechanism of Action • 2.2 Antidotes • 3 Classes • 3.1 G-Series • 3.2 V-Series • 3.3 Novichok Agents • 3.4 Insecticides • 4 History • 4.1 The Discovery ofNerve Agents • 4.2 The Nazi Mass Production ofTabun • 4.3 Nerve Agents in Nazi Germany • 4.4 The Secret Gets Out • 4.5 Since World War II • 4.6 Ocean Disposal of Chemical Weapons • 5 Popular Culture • 6 References and External Links --------------- ----·-- - Overview As chemical weapons, they are classified as weapons of mass destruction by the United Nations according to UN Resolution 687, and their production and stockpiling was outlawed by the Chemical Weapons Convention of 1993; the Chemical Weapons Convention officially took effect on April 291997. Poisoning by a nerve agent leads to contraction of pupils, profuse salivation, convulsions, involuntary urination and defecation, and eventual death by asphyxiation as control is lost over respiratory muscles. -

Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas
processes Article Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas Usman Shoukat , Diego D. D. Pinto and Hanna K. Knuutila * Department of Chemical Engineering, Norwegian University of Science and Technology (NTNU), 7491 Trondheim, Norway; [email protected] (U.S.); [email protected] (D.D.D.P.) * Correspondence: [email protected] Received: 8 February 2019; Accepted: 8 March 2019; Published: 15 March 2019 Abstract: Various novel amine solutions both in aqueous and non-aqueous [monoethylene glycol (MEG)/triethylene glycol(TEG)] forms have been studied for hydrogen sulfide (H2S) absorption. The study was conducted in a custom build experimental setup at temperatures relevant to subsea operation conditions and atmospheric pressure. Liquid phase absorbed H2S, and amine concentrations were measured analytically to calculate H2S loading (mole of H2S/mole of amine). Maximum achieved H2S loadings as the function of pKa, gas partial pressure, temperature and amine concentration are presented. Effects of solvent type on absorbed H2S have also been discussed. Several new solvents showed higher H2S loading as compared to aqueous N-Methyldiethanolamine (MDEA) solution which is the current industrial benchmark compound for selective H2S removal in natural gas sweetening process. Keywords: H2S absorption; amine solutions; glycols; desulfurization; aqueous and non-aqueous solutions 1. Introduction Natural gas is considered one of the cleanest forms of fossil fuel. Its usage in industrial processes and human activities is increasing worldwide, providing 23.4% of total world energy requirement in 2017 [1]. Natural gas is half of the price of crude oil and produces 29% less carbon dioxide than oil per unit of energy output [2]. -

Modern Chemical Weapons
Modern Chemical Weapons Modern Chemical Weapons causes serious diseases like cancer and serious birth defects in newly born Large scale chemical weapons were children. first used in World War One and have been used ever since. About 100 years ago Modern warfare has developed significantly since the early 20th century Early chemical weapons being used but the use of toxic chemicals to kill around a hundred years ago included: and badly injure is still very much in use tear gas, chlorine gas, mustard gas today. There have been reports of and phosgene gas. Since then, some chemical weapon attacks in Syria of the same chemicals have been during 2016. Chemical weapons have used in more modern warfare, but also been the choice of terrorists other new chemical weapons have because they are not very expensive also been developed. and need very little specialist knowledge to use them. These Chlorine gas (Cl2) weapons can cause a lot of causalities as well as fatalities, but also There have been reports of many spread panic and fear. chlorine gas attacks in Syria since 2013. It is a yellow-green gas which has a very distinctive smell like bleach. However, it does not last very long and therefore it is sometimes very difficult to prove it has been used during an attack. Victims would feel irritation of the eyes, nose, throat and lungs when they inhale it in large enough quantities. In even larger quantities it can cause the death of a person by suffocation. Mustard gas (C4H8Cl2S) There are reports by the United Nations (UN) of terrorist groups using Mustard Agent Orange (mixture of gas during chemical attacks in Syria. -

Nitrosamines EMEA-H-A5(3)-1490
25 June 2020 EMA/369136/2020 Committee for Medicinal Products for Human Use (CHMP) Assessment report Procedure under Article 5(3) of Regulation EC (No) 726/2004 Nitrosamine impurities in human medicinal products Procedure number: EMEA/H/A-5(3)/1490 Note: Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ...................................................................................... 2 1. Information on the procedure ............................................................... 7 2. Scientific discussion .............................................................................. 7 2.1. Introduction......................................................................................................... 7 2.2. Quality and safety aspects ..................................................................................... 7 2.2.1. Root causes for presence of N-nitrosamines in medicinal products and measures to mitigate them............................................................................................................. 8 2.2.2. Presence and formation of N-nitrosamines