Combustion and Pyrolysis Kinetics of Chloropicrin J.-C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Threat Agents Call Poison Control 24/7 for Treatment Information 1.800.222.1222 Blood Nerve Blister Pulmonary Metals Toxins
CHEMICAL THREAT AGENTS CALL POISON CONTROL 24/7 FOR TREATMENT INFORMATION 1.800.222.1222 BLOOD NERVE BLISTER PULMONARY METALS TOXINS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS • Vertigo • Diarrhea, diaphoresis • Itching • Upper respiratory tract • Cough • Shock • Tachycardia • Urination • Erythema irritation • Metallic taste • Organ failure • Tachypnea • Miosis • Yellowish blisters • Rhinitis • CNS effects • Cyanosis • Bradycardia, bronchospasm • Flu-like symptoms • Coughing • Shortness of breath • Flu-like symptoms • Emesis • Delayed eye irritation • Choking • Flu-like symptoms • Nonspecific neurological • Lacrimation • Delayed pulmonary edema • Visual disturbances symptoms • Salivation, sweating INDICATIVE LAB TESTS INDICATIVE LAB TESTS INDICATIVE LAB TEST INDICATIVE LAB TESTS INDICATIVE LAB TESTS INDICATIVE LAB TESTS • Increased anion gap • Decreased cholinesterase • Thiodiglycol present in urine • Decreased pO2 • Proteinuria None Available • Metabolic acidosis • Increased anion gap • Decreased pCO2 • Renal assessment • Narrow pO2 difference • Metabolic acidosis • Arterial blood gas between arterial and venous • Chest radiography samples DEFINITIVE TEST DEFINITIVE TEST DEFINITIVE TEST DEFINITIVE TESTS DEFINITIVE TESTS • Blood cyanide levels • Urine nerve agent • Urine blister agent No definitive tests available • Blood metals panel • Urine ricinine metabolites metabolites • Urine metals panel • Urine abrine POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS • Hydrogen Cyanide -

Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard
Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude To cite this version: Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas. Journal of Physical Chemistry A, American Chemical Society, 2017, 121 (17), pp.3254-3262. 10.1021/acs.jpca.7b01238. hal-01708219 HAL Id: hal-01708219 https://hal.archives-ouvertes.fr/hal-01708219 Submitted on 13 Feb 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta†, Baptiste Sirjean†, Laurent Verdier‡, René Fournet†, Pierre-Alexandre Glaude†,* †Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine, 1 rue Grandville BP 20451 54001 Nancy Cedex, France ‡DGA Maîtrise NRBC, Site du Bouchet, 5 rue Lavoisier, BP n°3, 91710 Vert le Petit, France *corresponding author: [email protected] Abstract The destruction of stockpiles or unexploded ammunitions of nitrogen mustard (tris (2- chloroethyl) amine, HN-3) requires the development of safe processes. -

Nerve Agent - Lntellipedia Page 1 Of9 Doc ID : 6637155 (U) Nerve Agent
This document is made available through the declassification efforts and research of John Greenewald, Jr., creator of: The Black Vault The Black Vault is the largest online Freedom of Information Act (FOIA) document clearinghouse in the world. The research efforts here are responsible for the declassification of MILLIONS of pages released by the U.S. Government & Military. Discover the Truth at: http://www.theblackvault.com Nerve Agent - lntellipedia Page 1 of9 Doc ID : 6637155 (U) Nerve Agent UNCLASSIFIED From lntellipedia Nerve Agents (also known as nerve gases, though these chemicals are liquid at room temperature) are a class of phosphorus-containing organic chemicals (organophosphates) that disrupt the mechanism by which nerves transfer messages to organs. The disruption is caused by blocking acetylcholinesterase, an enzyme that normally relaxes the activity of acetylcholine, a neurotransmitter. ...--------- --- -·---- - --- -·-- --- --- Contents • 1 Overview • 2 Biological Effects • 2.1 Mechanism of Action • 2.2 Antidotes • 3 Classes • 3.1 G-Series • 3.2 V-Series • 3.3 Novichok Agents • 3.4 Insecticides • 4 History • 4.1 The Discovery ofNerve Agents • 4.2 The Nazi Mass Production ofTabun • 4.3 Nerve Agents in Nazi Germany • 4.4 The Secret Gets Out • 4.5 Since World War II • 4.6 Ocean Disposal of Chemical Weapons • 5 Popular Culture • 6 References and External Links --------------- ----·-- - Overview As chemical weapons, they are classified as weapons of mass destruction by the United Nations according to UN Resolution 687, and their production and stockpiling was outlawed by the Chemical Weapons Convention of 1993; the Chemical Weapons Convention officially took effect on April 291997. Poisoning by a nerve agent leads to contraction of pupils, profuse salivation, convulsions, involuntary urination and defecation, and eventual death by asphyxiation as control is lost over respiratory muscles. -

Fumigation for Rabbit Control
Fumigation for rabbit control Introduction: Fumigation is a valuable tool in successful rabbit management programs. It is an effective follow-up technique to poison baiting and warren ripping, and is a particularly useful control method in areas where other techniques cannot be used (eg poison baiting). Fumigation works by replacing the air in warrens with Figure 1: Phostoxin tablets and dry paper are pushed deep lethal gasses, which are in-turn inhaled by rabbits, into the warren entrance. Image: Invasive Animals CRC causing them to suffocate and die. There are two types of fumigation: Fumigants Chloropicrin: Chloropicrin is a colourless, toxic liquid • pressure fumigation - where the fumigant is generated that is currently used in Australia as an insecticide, soil outside the warren and forced into the warren under and warren fumigant, and rodenticide. Chloropicrin pressure, usually from a pump is classified as a dangerous poison (Schedule 7) as • diffusion (or static) fumigation – where tablets are placed it has a high potential to cause harm, even at low in active burrows (Figure 1) and the fumigant generated exposures. Chloropicrin has recently been placed under is allowed to passively diffuse through the warren (this is review by the Australian Pesticides and Veterinary the preferred method)1. Medicines Authority (APVMA) because of environmental, There are strict regulations around the use of fumigants human health and human safety concerns. It is not for rabbit control that reduce the risk of harm to recommended for use, even though it is still registered operators. For this reason, users may also require training in some states. Pressure fumigation using chloropicrin ® or accreditation before using approved fumigants. -

Toxic Industrial Chemicals
J R Army Med Corps 2002; 148: 371-381 J R Army Med Corps: first published as 10.1136/jramc-148-04-06 on 1 December 2002. Downloaded from Toxic Industrial Chemicals Introduction location to another. Depending on the The first chemical warfare agent of the available routes of movement, and quantity modern era, chlorine, was released with of chemical to be moved, transport can occur devastating effect on 22 April 1915 at Ypres, by truck or rail tank cars, over water by barge Belgium. Along a 4 mile front, German or boat, over land through above- or below- soldiers opened the valves of 1,600 large and ground pipelines and by air. 4,130 small cylinders containing 168 tons of Toxic chemicals may be produced by the chlorine.The gas formed a thick white cloud burning of materials (e.g., the burning of that crossed the first allied trenches in less Teflon produces perfluoroisobutylene) or by than a minute.The allied line broke, allowing their reaction if spilled into water (e.g. silanes the Germans to advance deep into allied produce hydrogen chloride and cyanides, territory. If the Germans had been fully hydrogen cyanide). prepared to exploit this breakthrough, the course and possibly the outcome of WWI Toxic Industrial Chemicals may have been very different. (TICs) Chlorine is a commodity industrial A Toxic Industrial Chemical (TIC) is defined chemical with hundreds of legitimate uses; it as: is not a "purpose designed" chemical warfare an industrial chemical which has a LCt50 agent. Phosgene, another commodity value of less than 100,000 mg.min/m3 in industrial chemical, accounted for 80% of any mammalian species and is produced in the chemical fatalities during WWI. -

Piratox Sheet N5 Suffocating Agents and Phosphine
Edition of October 2011 Piratox sheet #5: "Suffocating agents and phosphine Indicative list of concerned agents: Toxic compounds with suffocating action - Phosgene (CAS number: 75-44-5) - Chlorine (CAS number: 7782-50-5) - Methyl isocyanate (CAS number: 624-83-9) - But also: o Ammonia (CAS number: 7664-41-7) o Diphosgene or surpalite (CAS number: 503-38-8) o Chloropicrin (CAS number: 76-06-2) o Fluorine (CAS number: 7782-41-4) o Perfluoroisobutylene (CAS number: 382-21-8) o Fumigants o Marketed industrial and domestic products - Phosphine or PH3 or Hydrogen Phosphide (CAS number: 7803-51-2). ! Key points not to forget The 1st emergency step is the extraction of victims from the hazard area: rescuers must possess suitable breathing and eye protection. At ambient temperature (20°C) most suffocating agents are gases that penetrate the body via the respiratory route. They are thus poorly or non-persistent, frequently limiting victim decontamination needs to simple undressing. Most suffocating agents are heavier than air. They affect the respiratory system: glottis, bronchi, alveoli and cause eye damage. In pre-hospital settings, avoid any physical exertion that could promote the onset of pulmonary oedema. In general, the shorter the symptom onset time, the more serious the intoxication and the more severe the symptoms. Treatment is symptomatic only. All symptomatic choking gas victims must encouraged to rest, in a sitting position, under oxygen. The duration of surveillance for symptomatic subjects is of at least 12 to 24 hours. For Phosphine (PH3): o victims must be undressed and showered; o toxicity is respiratory, cardiac, renal and neurological; o subjects having inhaled PH3 and presenting with significant initial manifestations shall be monitored in the hospital for 48 to 72 hours, due to the risk of delayed acute pulmonary oedema. -

Process for the Synthesis of N-Methyl-N-Phenylaminoacrolein
Europäisches Patentamt *EP001477474A1* (19) European Patent Office Office européen des brevets (11) EP 1 477 474 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 221/00, C07C 223/02 17.11.2004 Bulletin 2004/47 (21) Application number: 03425306.2 (22) Date of filing: 13.05.2003 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Banfi, Aldo HU IE IT LI LU MC NL PT RO SE SI SK TR 20131 Milano (IT) Designated Extension States: • Mancini, Alfredo AL LT LV MK 26020 Spinadesco (Cremona) (IT) (71) Applicant: Clariant Life Science Molecules (Italia) (74) Representative: Pistolesi, Roberto et al SpA Dragotti & Associati SRL 21040 Origgio (Varese) (IT) Galleria San Babila 4/c 20122 Milano (IT) (54) Process for the synthesis of N-methyl-N-phenylaminoacrolein (57) A process is disclosed for manufacturing N-me- thyl-N-phenylaminoacrolein of formula (I) wherein R is a C3-C4 alkyl, said process being charac- terized in that the reaction between N-methylformanilide and said alkyl vinyl ether of formula (III) is carried out in the presence of phosgene, diphosgene or triphosgene which comprises reacting N-methylformanilide and an in a solvent selected from dioxane, acetonitrile and/or alkyl vinyl ether of formula (III) chlorobenzene. EP 1 477 474 A1 Printed by Jouve, 75001 PARIS (FR) 1 EP 1 477 474 A1 2 Description [0003] More in details, compound (I) can be converted into Fenal, by reaction with compound (IV) [0001] This invention relates to the synthesis of N-me- thyl-N-phenylaminoacrolein of formula (I) 5 10 15 and its use for the preparation of 3-[3-(4-Fluorophenyl)- 1-(1-Methylethyl)-1H-Indol-2-yl]-2-Propenal (II), herein- after referred to as "Fenal", 20 in acetonitrile in the presence of POCl3, as disclosed in WO 84/82131 and US-4739073. -

3Cyanide and Fumigants February 2014 Hoffman
Cyanide & Fumigants Suzanne Doyon, MD, FACMT American College of Medical Toxicology Bethesda, MD, April 29, 2014 Chemical Agents of Opportunity 1 Faculty Disclosure • Faculty: Suzanne Doyon, MD – Relationships with commercial interests: none – Speakers Bureau/Honoraria: none – Consulting Fees: none – Other: none 2 Learning Objectives • Indicate the sources and uses of cyanide and fumigants • Describe therapies used to treat cyanide poisoning • List the four most common fumigant gases • Describe the clinical effects of exposure to these gases • Explain how to treat victims exposed to these gases 3 Cyanide & Fumigants • Cyanide – Salts (solids) – Gas • Fumigant gases – Vikane (sulfuryl fluoride) – Methyl bromide – Phosphine 4 Cyanide • Notoriety well deserved • Historical relevance – Mass poisoning • Pharmaceutical terrorism • Weapon of Mass Destruction 5 Cyanide (CN): Properties • Small molecule (26 Dalton) • Boiling Point 27.7°C • Colorless • Bitter Almonds? Myth • Water soluble 6 Cyanide: Two Common Forms Hydrogen Cyanide Solid Cyanide Salts Gas (sodium cyanide, potassium cyanide, calcium cyanide) Toxic when Inhaled Toxic when Ingested 7 Generating HCN Gas from Salts 8 Cyanide • Sources of cyanide (solid) – Industrial applications (electroplating, hardening steel, mining, fumigation,…) – Sodium, potassium and calcium cyanide are all readily purchased on the internet • Other sources – Cyanogen chloride – Acetonitrile, acrylonitrile – Natural occurring cyanogens (laetrile) 9 Cyanide: Mechanism of Action • Readily enters cells • Inhibits -

Toxic Exposures Kathy L
8 MODULE 8 Toxic Exposures Kathy L. Leham-Huskamp / William J. Keenan / Anthony J. Scalzo / Shan Yin 8 Toxic Exposures Kathy L. Lehman-Huskamp, MD William J. Keenan, MD Anthony J. Scalzo, MD Shan Yin, MD InTrODUcTIOn The first large-scale production of chemical and biological weapons occurred during the 20th century. World War I introduced the use of toxic gases such as chlorine, cyanide, an arsine as a means of chemical warfare. With recent events, such as the airplane attacks on the World Trade Center in New York City, people have become increasingly fearful of potential large-scale terrorist attacks. Consequently, there has been a heightened interest in disaster preparedness especially involving chemical and biological agents. The U.S. Federal Emergency Management Agency (FEMA) recommends an "all-hazards" approach to emergency planning. This means creating a simultaneous plan for intentional terrorist events as well as for the more likely unintentional public health emergencies, such as earthquakes, floods, hazardous chemical spills, and infectious outbreaks. Most large-scale hazardous exposures are determined by the type of major industries that exist and/or the susceptibility to different types of natural disasters in a given area. For example, in 1984 one of the greatest man-made disasters of all times occurred in Bhopal, India, when a Union Carbide pesticide plant released tons of methylisocyanate gas over a populated area, killing scores of thousands and injuring well over 250,000 individuals. The 2011 earthquake and tsunami in Japan demonstrated the vulnerability of nuclear power stations to natural disasters and the need to prepare for possible widespread nuclear contamination and radiation exposure. -

Table II. EPCRA Section 313 Chemical List for Reporting Year 2007 (Including Toxic Chemical Categories)
Table II. EPCRA Section 313 Chemical List For Reporting Year 2007 (including Toxic Chemical Categories) Individually listed EPCRA Section 313 chemicals with CAS numbers are arranged alphabetically starting on page II-3. Following the alphabetical list, the EPCRA Section 313 chemicals are arranged in CAS number order. Covered chemical categories follow. Certain EPCRA Section 313 chemicals listed in Table II have parenthetic “qualifiers.” These qualifiers indicate that these EPCRA Section 313 chemicals are subject to the section 313 reporting requirements if manufactured, processed, or otherwise used in a specific form or when a certain activity is performed. The following chemicals are reportable only if they are manufactured, processed, or otherwise used in the specific form(s) listed below: Chemical CAS Number Qualifier Aluminum (fume or dust) 7429-90-5 Only if it is a fume or dust form. Aluminum oxide (fibrous forms) 1344-28-1 Only if it is a fibrous form. Ammonia (includes anhydrous ammonia and aqueous ammonia 7664-41-7 Only 10% of aqueous forms. 100% of from water dissociable ammonium salts and other sources; 10 anhydrous forms. percent of total aqueous ammonia is reportable under this listing) Asbestos (friable) 1332-21-4 Only if it is a friable form. Hydrochloric acid (acid aerosols including mists, vapors, gas, 7647-01-0 Only if it is an aerosol form as fog, and other airborne forms of any particle size) defined. Phosphorus (yellow or white) 7723-14-0 Only if it is a yellow or white form. Sulfuric acid (acid aerosols including mists, vapors, gas, fog, and 7664-93-9 Only if it is an aerosol form as other airborne forms of any particle size) defined. -

Things to Be Done
DRAFT MAY 2003 ANNEX 1: CHEMICAL AGENTS 1. Introduction The large-scale use of toxic chemicals as weapons first became possible during the First World War (1914–1918) thanks to the growth of the chemical industry. More than 110 000 tonnes were disseminated over the battlefields, the greater part on the western front. Initially, the chemicals were used, not to cause casualties in the sense of putting enemy combatants out of action, but rather to harass. Though the sensory irritants used were powerful enough to disable those who were exposed to them, they served mainly to drive enemy combatants out of the trenches or other cover that protected them from conventional fire, or to disrupt enemy artillery or supplies. About 10% of the total tonnage of chemical warfare agents used during the war were chemicals of this type, namely lacrimators (tear gases), sternutators and vomiting agents. However, use of more lethal chemicals soon followed the introduction of disabling chemicals. In all, chemical agents caused some 1.3 million casualties, including 90 000 deaths. During the First World War, almost every known noxious chemical was screened for its potential as a weapon, and this process was repeated during the Second World War (1939–1945), when substantial stocks of chemical weapons were accumulated, although rarely used in military operations. Between the two world wars, a high proportion of all the new compounds that had been synthesized, or isolated from natural materials, were examined to determine their utility as lethal or disabling chemical weapons. After 1945, these systematic surveys continued, together with a search for novel agents based on advances in biochemistry, toxicology and pharmacology. -
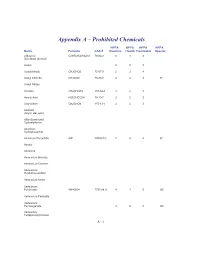
Appendix a – Prohibited Chemicals
Appendix A – Prohibited Chemicals NFPA NFPA NFPA NFPA Name Formula CAS #Reactive Health Flammable Special 2-Butanol C2H5CH(OH)CH3 78-92-2 0 1 3 (Sec-Butyl Alcohol) Acetal 023 Acetaldehyde CH3CHO5 75-07-0 2 3 4 Acetyl Chloride CH3COCl 75-36-5 2 3 3 W Acetyl Nitrate Acrolein CH2CHCHO 107-02-8 3 4 3 Acrylic Acid H2CCHCO2H 79-10-7 2 2 2 Acrylonitrile CH2CHCN 107-13-1 2 4 3 Alcohols (Allylic, Benzylic) Alkly-Substituted Cycloaliphatics Aluminum Hydrophosphide Aluminum Phosphide AlP 20859-73- 2 4 4 W Amatol Ammonal Ammonium Bromate Ammonium Chlorate Ammonium Hexanitrocobaltate Ammonium Nitrite Ammonium Perchlorate NH4ClO4 7790-98-9 4 1 0 OX Ammonium Periodate Ammonium Permanganate 300 OX Ammonium Tetraperoxychromate A - 1 Appendix A – Prohibited Chemicals NFPA NFPA NFPA NFPA Name Formula CAS #Reactive Health Flammable Special Antimony Compounds Arsenic And Arsenic Compounds Azides Azidocarbonyl Guanidine Barium Ba 2 2 1 W Barium Chlorate Ba(ClO3)2*H2O 13477-00- 1 2 0 OX Barium Oxide (Anhydrous) BaO 1304-28-5 2 3 0 Barium Peroxide BaO2 1304-29-6 0 1 0 OX Benzene C6H6 71-43-2 0 2 3 Benzene Diazonium Chloride Benzotriazole C6H5N3 95-14-7 0 2 1 Benzoyl Peroxide (C6H5CO)2O2 94-36-0 4 1 4 OX Benzyl Alcohol C6H5CH2OH 100-51-6 0 2 1 Bismuth Nitrate Bi(NO3)3*5H2O 10035-06- 3 1 0 OX Borane,Boranes, Diboranes Boron Tribromide 230 W Boron Trifluoride 140 Bromine Pentafluoride Brf5 7789-30-2 3 4 0 W,O Bromine Trifluoride 3 4 0 W,O Butadiene C4H6/CH2=(CH)2=CH 106-99-0 0 2 4 Butenetroil Trinitrate Cadmium and Cadmium Compounds Calcium Nitrate, Anhydrous Ca(NO3)2