Chloropicrin for Possible Carcinogenicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard
Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude To cite this version: Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas. Journal of Physical Chemistry A, American Chemical Society, 2017, 121 (17), pp.3254-3262. 10.1021/acs.jpca.7b01238. hal-01708219 HAL Id: hal-01708219 https://hal.archives-ouvertes.fr/hal-01708219 Submitted on 13 Feb 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta†, Baptiste Sirjean†, Laurent Verdier‡, René Fournet†, Pierre-Alexandre Glaude†,* †Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine, 1 rue Grandville BP 20451 54001 Nancy Cedex, France ‡DGA Maîtrise NRBC, Site du Bouchet, 5 rue Lavoisier, BP n°3, 91710 Vert le Petit, France *corresponding author: [email protected] Abstract The destruction of stockpiles or unexploded ammunitions of nitrogen mustard (tris (2- chloroethyl) amine, HN-3) requires the development of safe processes. -

Nerve Agent - Lntellipedia Page 1 Of9 Doc ID : 6637155 (U) Nerve Agent
This document is made available through the declassification efforts and research of John Greenewald, Jr., creator of: The Black Vault The Black Vault is the largest online Freedom of Information Act (FOIA) document clearinghouse in the world. The research efforts here are responsible for the declassification of MILLIONS of pages released by the U.S. Government & Military. Discover the Truth at: http://www.theblackvault.com Nerve Agent - lntellipedia Page 1 of9 Doc ID : 6637155 (U) Nerve Agent UNCLASSIFIED From lntellipedia Nerve Agents (also known as nerve gases, though these chemicals are liquid at room temperature) are a class of phosphorus-containing organic chemicals (organophosphates) that disrupt the mechanism by which nerves transfer messages to organs. The disruption is caused by blocking acetylcholinesterase, an enzyme that normally relaxes the activity of acetylcholine, a neurotransmitter. ...--------- --- -·---- - --- -·-- --- --- Contents • 1 Overview • 2 Biological Effects • 2.1 Mechanism of Action • 2.2 Antidotes • 3 Classes • 3.1 G-Series • 3.2 V-Series • 3.3 Novichok Agents • 3.4 Insecticides • 4 History • 4.1 The Discovery ofNerve Agents • 4.2 The Nazi Mass Production ofTabun • 4.3 Nerve Agents in Nazi Germany • 4.4 The Secret Gets Out • 4.5 Since World War II • 4.6 Ocean Disposal of Chemical Weapons • 5 Popular Culture • 6 References and External Links --------------- ----·-- - Overview As chemical weapons, they are classified as weapons of mass destruction by the United Nations according to UN Resolution 687, and their production and stockpiling was outlawed by the Chemical Weapons Convention of 1993; the Chemical Weapons Convention officially took effect on April 291997. Poisoning by a nerve agent leads to contraction of pupils, profuse salivation, convulsions, involuntary urination and defecation, and eventual death by asphyxiation as control is lost over respiratory muscles. -

Fumigation for Rabbit Control
Fumigation for rabbit control Introduction: Fumigation is a valuable tool in successful rabbit management programs. It is an effective follow-up technique to poison baiting and warren ripping, and is a particularly useful control method in areas where other techniques cannot be used (eg poison baiting). Fumigation works by replacing the air in warrens with Figure 1: Phostoxin tablets and dry paper are pushed deep lethal gasses, which are in-turn inhaled by rabbits, into the warren entrance. Image: Invasive Animals CRC causing them to suffocate and die. There are two types of fumigation: Fumigants Chloropicrin: Chloropicrin is a colourless, toxic liquid • pressure fumigation - where the fumigant is generated that is currently used in Australia as an insecticide, soil outside the warren and forced into the warren under and warren fumigant, and rodenticide. Chloropicrin pressure, usually from a pump is classified as a dangerous poison (Schedule 7) as • diffusion (or static) fumigation – where tablets are placed it has a high potential to cause harm, even at low in active burrows (Figure 1) and the fumigant generated exposures. Chloropicrin has recently been placed under is allowed to passively diffuse through the warren (this is review by the Australian Pesticides and Veterinary the preferred method)1. Medicines Authority (APVMA) because of environmental, There are strict regulations around the use of fumigants human health and human safety concerns. It is not for rabbit control that reduce the risk of harm to recommended for use, even though it is still registered operators. For this reason, users may also require training in some states. Pressure fumigation using chloropicrin ® or accreditation before using approved fumigants. -

Combustion and Pyrolysis Kinetics of Chloropicrin J.-C
Combustion and Pyrolysis Kinetics of Chloropicrin J.-C. Lizardo-Huerta, B. Sirjean, L. Verdier, R. Fournet, Pierre-Alexandre Glaude To cite this version: J.-C. Lizardo-Huerta, B. Sirjean, L. Verdier, R. Fournet, Pierre-Alexandre Glaude. Combustion and Pyrolysis Kinetics of Chloropicrin. Journal of Physical Chemistry A, American Chemical Society, 2018, 122 (26), pp.5735 - 5741. 10.1021/acs.jpca.8b04007. hal-01921757 HAL Id: hal-01921757 https://hal.univ-lorraine.fr/hal-01921757 Submitted on 14 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Combustion and Pyrolysis Kinetics of Chloropicrin J.-C. Lizardo-Huerta1, B. Sirjean1, L. Verdier2, R. Fournet1, P.-A. Glaude1* 1 Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine 1 rue Grandville BP 20451 54001 Nancy Cedex, France 2 DGA Maîtrise NRBC, Site du Bouchet, 5 rue Lavoisier, BP n°3, 91710 Vert le Petit, France Corresponding author : Pierre-Alexandre Glaude Laboratoire Réactions et Génie des Procédés 1 rue Grandville BP 20451 54001 Nancy Cedex, France Email:[email protected] 1 Abstract Chloropicrin (CCl3NO2) is widely used in agriculture as a pesticide, weed-killer, fungicide or nematicide. -

3Cyanide and Fumigants February 2014 Hoffman
Cyanide & Fumigants Suzanne Doyon, MD, FACMT American College of Medical Toxicology Bethesda, MD, April 29, 2014 Chemical Agents of Opportunity 1 Faculty Disclosure • Faculty: Suzanne Doyon, MD – Relationships with commercial interests: none – Speakers Bureau/Honoraria: none – Consulting Fees: none – Other: none 2 Learning Objectives • Indicate the sources and uses of cyanide and fumigants • Describe therapies used to treat cyanide poisoning • List the four most common fumigant gases • Describe the clinical effects of exposure to these gases • Explain how to treat victims exposed to these gases 3 Cyanide & Fumigants • Cyanide – Salts (solids) – Gas • Fumigant gases – Vikane (sulfuryl fluoride) – Methyl bromide – Phosphine 4 Cyanide • Notoriety well deserved • Historical relevance – Mass poisoning • Pharmaceutical terrorism • Weapon of Mass Destruction 5 Cyanide (CN): Properties • Small molecule (26 Dalton) • Boiling Point 27.7°C • Colorless • Bitter Almonds? Myth • Water soluble 6 Cyanide: Two Common Forms Hydrogen Cyanide Solid Cyanide Salts Gas (sodium cyanide, potassium cyanide, calcium cyanide) Toxic when Inhaled Toxic when Ingested 7 Generating HCN Gas from Salts 8 Cyanide • Sources of cyanide (solid) – Industrial applications (electroplating, hardening steel, mining, fumigation,…) – Sodium, potassium and calcium cyanide are all readily purchased on the internet • Other sources – Cyanogen chloride – Acetonitrile, acrylonitrile – Natural occurring cyanogens (laetrile) 9 Cyanide: Mechanism of Action • Readily enters cells • Inhibits -

Table II. EPCRA Section 313 Chemical List for Reporting Year 2007 (Including Toxic Chemical Categories)
Table II. EPCRA Section 313 Chemical List For Reporting Year 2007 (including Toxic Chemical Categories) Individually listed EPCRA Section 313 chemicals with CAS numbers are arranged alphabetically starting on page II-3. Following the alphabetical list, the EPCRA Section 313 chemicals are arranged in CAS number order. Covered chemical categories follow. Certain EPCRA Section 313 chemicals listed in Table II have parenthetic “qualifiers.” These qualifiers indicate that these EPCRA Section 313 chemicals are subject to the section 313 reporting requirements if manufactured, processed, or otherwise used in a specific form or when a certain activity is performed. The following chemicals are reportable only if they are manufactured, processed, or otherwise used in the specific form(s) listed below: Chemical CAS Number Qualifier Aluminum (fume or dust) 7429-90-5 Only if it is a fume or dust form. Aluminum oxide (fibrous forms) 1344-28-1 Only if it is a fibrous form. Ammonia (includes anhydrous ammonia and aqueous ammonia 7664-41-7 Only 10% of aqueous forms. 100% of from water dissociable ammonium salts and other sources; 10 anhydrous forms. percent of total aqueous ammonia is reportable under this listing) Asbestos (friable) 1332-21-4 Only if it is a friable form. Hydrochloric acid (acid aerosols including mists, vapors, gas, 7647-01-0 Only if it is an aerosol form as fog, and other airborne forms of any particle size) defined. Phosphorus (yellow or white) 7723-14-0 Only if it is a yellow or white form. Sulfuric acid (acid aerosols including mists, vapors, gas, fog, and 7664-93-9 Only if it is an aerosol form as other airborne forms of any particle size) defined. -
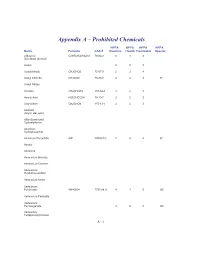
Appendix a – Prohibited Chemicals
Appendix A – Prohibited Chemicals NFPA NFPA NFPA NFPA Name Formula CAS #Reactive Health Flammable Special 2-Butanol C2H5CH(OH)CH3 78-92-2 0 1 3 (Sec-Butyl Alcohol) Acetal 023 Acetaldehyde CH3CHO5 75-07-0 2 3 4 Acetyl Chloride CH3COCl 75-36-5 2 3 3 W Acetyl Nitrate Acrolein CH2CHCHO 107-02-8 3 4 3 Acrylic Acid H2CCHCO2H 79-10-7 2 2 2 Acrylonitrile CH2CHCN 107-13-1 2 4 3 Alcohols (Allylic, Benzylic) Alkly-Substituted Cycloaliphatics Aluminum Hydrophosphide Aluminum Phosphide AlP 20859-73- 2 4 4 W Amatol Ammonal Ammonium Bromate Ammonium Chlorate Ammonium Hexanitrocobaltate Ammonium Nitrite Ammonium Perchlorate NH4ClO4 7790-98-9 4 1 0 OX Ammonium Periodate Ammonium Permanganate 300 OX Ammonium Tetraperoxychromate A - 1 Appendix A – Prohibited Chemicals NFPA NFPA NFPA NFPA Name Formula CAS #Reactive Health Flammable Special Antimony Compounds Arsenic And Arsenic Compounds Azides Azidocarbonyl Guanidine Barium Ba 2 2 1 W Barium Chlorate Ba(ClO3)2*H2O 13477-00- 1 2 0 OX Barium Oxide (Anhydrous) BaO 1304-28-5 2 3 0 Barium Peroxide BaO2 1304-29-6 0 1 0 OX Benzene C6H6 71-43-2 0 2 3 Benzene Diazonium Chloride Benzotriazole C6H5N3 95-14-7 0 2 1 Benzoyl Peroxide (C6H5CO)2O2 94-36-0 4 1 4 OX Benzyl Alcohol C6H5CH2OH 100-51-6 0 2 1 Bismuth Nitrate Bi(NO3)3*5H2O 10035-06- 3 1 0 OX Borane,Boranes, Diboranes Boron Tribromide 230 W Boron Trifluoride 140 Bromine Pentafluoride Brf5 7789-30-2 3 4 0 W,O Bromine Trifluoride 3 4 0 W,O Butadiene C4H6/CH2=(CH)2=CH 106-99-0 0 2 4 Butenetroil Trinitrate Cadmium and Cadmium Compounds Calcium Nitrate, Anhydrous Ca(NO3)2 -

Module Five: Cyanide & Fumigants
Chemical Agents of Opportunity for Terrorism: TICs & TIMs Module Five: Cyanide & Fumigants Paul Wax, MD, FACMT American College of Medical Toxicology 1 Chemical Agents of Opportunity for Terrorism: TICs & TIMs Course Overview 1. Introduction / Principles of Medical Toxicology 2. Why Toxic Industrial Chemicals as Terrorist Weapons? 3. Inhalation of Toxic Industrial Gases 4. Agricultural Chemicals of Concern 5. Cyanide and Fumigants 6. Psychological Consequences of Mass Chemical Exposure 7. Risk Communication 8. Neurotoxins 9. Water, Food & Medication as Vectors 10. Delayed-Onset Toxins 11. Post-Event Medical Monitoring 12. Tabletop Exercise Module Five – Cyanide and Fumigants 2 Chemical Agents of Opportunity for Terrorism: TICs & TIMs Please help us improve this course by filling out the module evaluation. You will receive an email with instructions following the conclusion of this presentation. Module Five – Cyanide and Fumigants 3 Chemical Agents of Opportunity for Terrorism: TICs & TIMs Faculty Disclosure • Faculty: Paul Wax, MD, FACMT – Relationships with commercial interests: none – Speakers Bureau/Honoraria: none – Consulting Fees: none – Other: none Module Five – Cyanide and Fumigants 4 Chemical Agents of Opportunity for Terrorism: TICs & TIMs Participant Question: • How many people are in attendance at your site (including yourself)? Module Five – Cyanide and Fumigants 5 Chemical Agents of Opportunity for Terrorism: TICs & TIMs Learning Objectives • Indicate the sources and uses of cyanide and fumigants • Describe therapies used -

Recognition and Management of Pesticide Poisonings: Sixth Edition: 2013: Chapter 17 Fumigants
CHAPTER 17 HIGHLIGHTS Easily absorbed in lung, gut, skin Fumigants SIGNS & SYMPTOMS Packaging and formulation of fumigants are complex. Those that are gases at room Highly variable among temperature (methyl bromide, ethylene oxide, sulfur dioxide, sulfuryl fluoride) are agents provided in compressed gas cylinders. Liquids are marketed in cans or drums. Solids that sublime, such as naphthalene, must be packaged so as to prevent significant Many are irritants contact with air before they are used. Sodium cyanide is only available in an encap- Carbon disulfide, chloroform, sulated form so that when wild canids attack livestock their bite releases the poison. ethylene dichloride, Mixtures of fumigants are sometimes used. For instance, chloropicrin, which has hydrogen cyanide, methyl a strong odor and irritant effect, is often added as a “warning agent” to other liquid bromide may have serious fumigants. It is important to be aware of the possibility of such mixtures. CNS effects Liquid halocarbons and carbon disulfide evaporate into the air while naphtha- lene sublimes. Paraformaldehyde slowly depolymerizes to formaldehyde. Aluminum Methyl bromide, ethylene phosphide slowly reacts with water vapor in the air to liberate phosphine, an extremely dibromide, ethylene oxide, toxic gas. aluminum phosphide Fumigants have remarkable capacities for diffusion (a property essential to (phosphine gas) can cause their function). Some readily penetrate rubber and neoprene personal protective gear, pulmonary edema as well as human skin. They are rapidly absorbed across the pulmonary membranes, Chloroform, carbon gastrointestinal tract and skin. Special adsorbents are required in respirator canisters tetrachloride, ethylene to protect exposed workers from airborne fumigant gases. Even these may not provide dichloride, ethylene complete protection when air concentrations of fumigants are high. -

Review of Chloropicrin: a Fumigant, Another Environmental Pollutant
International Journal of Latest Trends in Engineering and Technology Vol.(8)Issue(4), pp.213-215 DOI: http://dx.doi.org/10.21172/1.84.35 e-ISSN:2278-621X REVIEW OF CHLOROPICRIN A FUMIGANT ANOTHER ENVIRONMENTAL POLLUTANT Jyoti Kumari1 Abstract-Chloropicrin is a chemical compound used to kill fungi, nematodes, microbes present in soil. Thereby it increases the productivity in agriculture. Chloropicrin is useful but it is harmful like other pesticides (DDT) because it acts as a pollutant. Its emission in environment increases the pollution level in air. In This article we discuss properties of chloropicrin and its relation with formation of ozone, a pollutant in troposphere. Keywords-Herbicide, Pollution, Troposphere, Photolysis, Free-radical, Experiment chamber, Organic compounds. 1. INTRODUCTION Chloropicrin is also known as trichloro nitro methane whose structure can be written as Chloropicrin is a chemical compound currently used as abroad spectrum antimicrobial, fungicide and herbicide. Initially it has been used as chemical weapon agent during world war. Chloropicrin is used as a reagent in the synthesis of organic chemicals, in the manufacture of methyl violet, and as a fumigant, it has also been used in chemical warfare agent. it is used as tear gas due its strong irritating nature. Sometime it is used as warning agent for fumigant methyl bromide and hydrogen cyanide (HCN). 2. PROPERTIES OF CHLOROPICRIN 1. Chloropicrin is highly volatile and due to this property it becomes a source of air pollution. 2. It acts as a powerful tear gas. 3. It has a strong odour so it is used as a warning agent when added in small amount to other fumigants such as HCN and CH3Br. -

TIH/PIH List
Hazardous Materials Designated as TIH/PIH (consolidated AAR and Railinc lists) 3/12/2007 STCC Proper Shipping Name 4921402 2-CHLOROETHANAL 4921495 2-METHYL-2-HEPTANETHIOL 4921741 3,5-DICHLORO-2,4,6-TRIFLUOROPYRIDINE 4921401 ACETONE CYANOHYDRIN, STABILIZED 4927007 ACROLEIN, STABILIZED 4921019 ALLYL ALCOHOL 4923113 ALLYL CHLOROFORMATE 4921004 ALLYLAMINE 4904211 AMMONIA SOLUTION 4920360 AMMONIA SOLUTIONS 4904209 AMMONIA, ANHYDROUS 4904210 AMMONIA, ANHYDROUS 4904879 AMMONIA, ANHYDROUS 4920359 AMMONIA, ANHYDROUS 4923209 ARSENIC TRICHLORIDE 4920135 ARSINE 4932010 BORON TRIBROMIDE 4920349 BORON TRICHLORIDE 4920522 BORON TRIFLUORIDE 4936110 BROMINE 4920715 BROMINE CHLORIDE 4918505 BROMINE PENTAFLUORIDE 4936106 BROMINE SOLUTIONS 4918507 BROMINE TRIFLUORIDE 4921727 BROMOACETONE 4920343 CARBON MONOXIDE AND HYDROGEN MIXTURE, COMPRESSED 4920399 CARBON MONOXIDE, COMPRESSED 4920511 CARBON MONOXIDE, REFRIGERATED LIQUID 4920559 CARBONYL FLUORIDE 4920351 CARBONYL SULFIDE 4920523 CHLORINE 4920189 CHLORINE PENTAFLUORIDE 4920352 CHLORINE TRIFLUORIDE 4921558 CHLOROACETONE, STABILIZED 4921009 CHLOROACETONITRILE 4923117 CHLOROACETYL CHLORIDE 4921414 CHLOROPICRIN 4920516 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920547 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920392 CHLOROPICRIN AND METHYL CHLORIDE MIXTURES 4921746 CHLOROPIVALOYL CHLORIDE 4930204 CHLOROSULFONIC ACID 4920527 COAL GAS, COMPRESSED 4920102 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920303 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920304 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920305 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920101 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920300 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920301 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920324 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920331 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920165 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920378 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920379 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. -

COMPARISON of FUMIGANT GASES USED for RABBIT CONTROL in GREAT BRITAIN JOHN ROSS, Mlntstjy Ofagricultural
COMPARISON OF FUMIGANT GASES USED FOR RABBIT CONTROL IN GREAT BRITAIN JOHN ROSS, MlntstJy ofAgricultural. Fisheries and Food, Agricultural Science Service. Guildford, England. ABSTRACT: The two most co1T1T1only used fumigant forrTl.llations, one generating hydrogen cyanide (HCN) and the other phosphine (PH3), were compared in paired field trials using the spoon-gassing technique. The two forrTl.llations were equally effective in reducing rabbit numbers seen in spotlight counts. The PH 3 generating fonnulation was more convenient and slightly cheaper to use . Safety and humaneness aspects of the two formulations are discussed and alternative fonnulations (generating HCN and PH 3) are consid ered. The potential usefulness (for rabbit control) of some other fumigant gases is briefly reviewed. INTRODUCTION The European rabbit (Or1ctolagus cuniculus) is the major vertebrate pest of British agriculture, currently causing damage est mated to cost tens of millions of pounds each year (Rees et al. 1985) . Rabbits were a serious pest during the first half of this century, but the arrival of myxomatosi s in 1953 led within two years to a 99% reduction in rabbit numbers (Lloyd 1970). Although rabbit numbers gradually built up again, economic rabbit damage to agriculture was negligible until the mid-1970s. Since then, rabbit numbers have increased more quickly, and it is estimated that nationally the rabbit population is now about 20% of the pre-myxomatosis population (Lloyd 1981). In some areas,, however, rabbit densities are well above the national average and crop loss is again serious. As rabbit numbers have increased, the need for effective control has also increased, but the methods of rabbit control available in Britain have changed little for many years.