The Search for Superheavy Elements: Historical and Philosophical Perspectives 1. Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
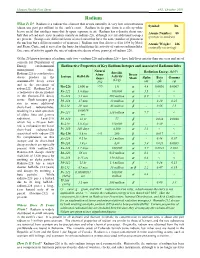
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

Table 2.Iii.1. Fissionable Isotopes1
FISSIONABLE ISOTOPES Charles P. Blair Last revised: 2012 “While several isotopes are theoretically fissionable, RANNSAD defines fissionable isotopes as either uranium-233 or 235; plutonium 238, 239, 240, 241, or 242, or Americium-241. See, Ackerman, Asal, Bale, Blair and Rethemeyer, Anatomizing Radiological and Nuclear Non-State Adversaries: Identifying the Adversary, p. 99-101, footnote #10, TABLE 2.III.1. FISSIONABLE ISOTOPES1 Isotope Availability Possible Fission Bare Critical Weapon-types mass2 Uranium-233 MEDIUM: DOE reportedly stores Gun-type or implosion-type 15 kg more than one metric ton of U- 233.3 Uranium-235 HIGH: As of 2007, 1700 metric Gun-type or implosion-type 50 kg tons of HEU existed globally, in both civilian and military stocks.4 Plutonium- HIGH: A separated global stock of Implosion 10 kg 238 plutonium, both civilian and military, of over 500 tons.5 Implosion 10 kg Plutonium- Produced in military and civilian 239 reactor fuels. Typically, reactor Plutonium- grade plutonium (RGP) consists Implosion 40 kg 240 of roughly 60 percent plutonium- Plutonium- 239, 25 percent plutonium-240, Implosion 10-13 kg nine percent plutonium-241, five 241 percent plutonium-242 and one Plutonium- percent plutonium-2386 (these Implosion 89 -100 kg 242 percentages are influenced by how long the fuel is irradiated in the reactor).7 1 This table is drawn, in part, from Charles P. Blair, “Jihadists and Nuclear Weapons,” in Gary A. Ackerman and Jeremy Tamsett, ed., Jihadists and Weapons of Mass Destruction: A Growing Threat (New York: Taylor and Francis, 2009), pp. 196-197. See also, David Albright N 2 “Bare critical mass” refers to the absence of an initiator or a reflector. -

R Epor T Resumes
R EPOR TRESUMES ED 010 991 SE 000 019 BIOLOGICAL EDUCATION IN AMERICAN SECONDARY SCHOOLS 1890-1960. BY- HURD, PAUL DEHART AMERICAN INST. OF BIOLOGICAL SCIENCES REPORT NUMBER BSCS BULLI PUB DATE 1 FEB 61 EDRS PRICEMF S0.45 HC$10.76 269P. DESCRIPTORS *BIOLOGY, *SCIENCE EDUCATION, *SECONDARY SCHOOL SCIENCE, CURRICULUM, COURSE CONTENT, EDUCATIONAL OBJECTIVES, HISTORY, TEACHING METHODS, TEXTBOOKS, BIOLOGICAL SCIENCES CURRICULUM STUDY, NATIONAL SCIENCE FOUNDATION, AMERICAN INSTITUTE OF BIOLOGICAL SCIENCES, DISTRICT OF COLUMBIA CHANGES IN AMERICAN SECONDARY SCHOOL BIOLOGICAL EDUCATION CURING THE PERIOD 1890-1960 ARE DESCRIBED. INFORMATION FROM THE REPORTS OF IMPORTANT COMMITTEES SUMMARIZES CHANGES IN BIOLOGICAL EDUCATION CURING EACH DECADE OF THE PERIOD COVERED BY THE STUDY. CHANGES IN COURSE CONTENT, TEACHING METHODOLOGY, AND RATIONALE ARE RELATED TO CORRESPONDING CHANGES IN SOCIAL STRUCTURE, EDUCATIONAL PHILOSOPHY, AND EXISTING KNOWLEDGE OF LEARNING THEORY. TOPICAL AREeeS ANALYZED INCLUDE (1) COURSE OBJECTIVES, (2) CRITERIA FOR THE SELECTION CF COURSE CONTENT, (3) TEXTBOOKS, (4) THE LEARNING CF BIOLOGY, AND (5) INSTRUCTIONAL RESOURCES. UNRESOLVED PROBLEMS IN BIOLOGICAL EDUCATION AND PROBLEMS AND ISSUES IN TEACHING BIOLOGY ARE DISCUSSED. (AG) op- lb" A II1111 It -41111thz- ,...11100t'. BIOLOGICAL SCIENCES CURRICULUM STUDY BULLETIN NO. 1 BIOLOGICAL ir(kryi( EDUCATION IN AMERICAN SECONDARY SCHOOLS 1890-1960 By Paul DeHart Hurd Education Consultant Ilielogical Sciences Curriculum Study On leave, School of Education Stanford University U.S. DEMMER Of RAM, MOON a WEIFAR ma Of MANN 11115 DEMI HAS Ka REPEONCEN UAW AS NaiveIRON Mt PERSON OR 01111111ATION OMANI IT.POINTS Of MEW OR OPINIONS STAND 00 NOT NECESSARY MUER OffKIAL OffKE OfEDUCATION POSITION 01 POUCY. American Institute of liological Sciences 2000 P Street, N.W. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

An Octad for Darmstadtium and Excitement for Copernicium
SYNOPSIS An Octad for Darmstadtium and Excitement for Copernicium The discovery that copernicium can decay into a new isotope of darmstadtium and the observation of a previously unseen excited state of copernicium provide clues to the location of the “island of stability.” By Katherine Wright holy grail of nuclear physics is to understand the stability uncover its position. of the periodic table’s heaviest elements. The problem Ais, these elements only exist in the lab and are hard to The team made their discoveries while studying the decay of make. In an experiment at the GSI Helmholtz Center for Heavy isotopes of flerovium, which they created by hitting a plutonium Ion Research in Germany, researchers have now observed a target with calcium ions. In their experiments, flerovium-288 previously unseen isotope of the heavy element darmstadtium (Z = 114, N = 174) decayed first into copernicium-284 and measured the decay of an excited state of an isotope of (Z = 112, N = 172) and then into darmstadtium-280 (Z = 110, another heavy element, copernicium [1]. The results could N = 170), a previously unseen isotope. They also measured an provide “anchor points” for theories that predict the stability of excited state of copernicium-282, another isotope of these heavy elements, says Anton Såmark-Roth, of Lund copernicium. Copernicium-282 is interesting because it University in Sweden, who helped conduct the experiments. contains an even number of protons and neutrons, and researchers had not previously measured an excited state of a A nuclide’s stability depends on how many protons (Z) and superheavy even-even nucleus, Såmark-Roth says. -

UC Irvine UC Irvine Previously Published Works
UC Irvine UC Irvine Previously Published Works Title Astrophysics in 2006 Permalink https://escholarship.org/uc/item/5760h9v8 Journal Space Science Reviews, 132(1) ISSN 0038-6308 Authors Trimble, V Aschwanden, MJ Hansen, CJ Publication Date 2007-09-01 DOI 10.1007/s11214-007-9224-0 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Space Sci Rev (2007) 132: 1–182 DOI 10.1007/s11214-007-9224-0 Astrophysics in 2006 Virginia Trimble · Markus J. Aschwanden · Carl J. Hansen Received: 11 May 2007 / Accepted: 24 May 2007 / Published online: 23 October 2007 © Springer Science+Business Media B.V. 2007 Abstract The fastest pulsar and the slowest nova; the oldest galaxies and the youngest stars; the weirdest life forms and the commonest dwarfs; the highest energy particles and the lowest energy photons. These were some of the extremes of Astrophysics 2006. We attempt also to bring you updates on things of which there is currently only one (habitable planets, the Sun, and the Universe) and others of which there are always many, like meteors and molecules, black holes and binaries. Keywords Cosmology: general · Galaxies: general · ISM: general · Stars: general · Sun: general · Planets and satellites: general · Astrobiology · Star clusters · Binary stars · Clusters of galaxies · Gamma-ray bursts · Milky Way · Earth · Active galaxies · Supernovae 1 Introduction Astrophysics in 2006 modifies a long tradition by moving to a new journal, which you hold in your (real or virtual) hands. The fifteen previous articles in the series are referenced oc- casionally as Ap91 to Ap05 below and appeared in volumes 104–118 of Publications of V. -

By Government, Particularly in Administrative Positions, and Exert an Increasing Demand and Suggest Future Action for Trained Pu
DOCUMENT RESUME ED 025 220 24 HE 000 314 By-Mosher, Frederick C. Professional Education and the Public Service; An Exploratory Study. Final Report. California Univ., Berkeley. Center for Research and Development in Higher Education. Spons Agency-Office of Education (DHEW), Washington, D.C. Bureau of Research Bureau No- BR-5-0248 Pub Date Apr 68 Contract- OEC- 6- 10- 106 Note-170p. EDRS Price MF-$0.75 HC-$8.60 Descriptors- Administrative Personnel, *Government (Administrative Body), Governmental Structure, *Government Emplo_yees, *Higher Education, Professional Education, Professional Occupations,Professional Personnel, Public Administration Education, Public Officials, *Public Policy Professional and technical fields are the fastest growing occupational sectors in the US. More than one-third of all professional and technical workers are employed by government, particularlyinadministrative positions, and exert an increasing influence on public policy. But, with the exception of city managers, many of these employees regard themselves as members of the disciplines in which they weretrained rather than primarily as public administrators. The field of public administrationhas not influenced public policy as much as other fields have, possibly because: it is not a specialized profession; practitioners do not agree on what its core knowledge,skills and orientation should be; and there is little incentive for students to pursue studies in the field since most administrative positions are held by otherspecialists. Althou9h more attention in colleges is now being paid toadministrative and management fields, courses are usually based onorganizational theories of private business models, with little emphasis on the unique problems of government administration.Professional schools suffer from a general bias against teaching the kinds of subjects thatmight be useful in government jobs. -

Gas-Phase Chemistry of Element 114, Flerovium
EPJ Web of Conferences 131, 07003 (2016) DOI: 10.1051/epjconf/201613107003 Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements Gas-phase chemistry of element 114, flerovium Alexander Yakushev1,2,a and Robert Eichler3,4 1 GSI Helmholtzzentrum für Schwerionenforschung GmbH, 64291 Darmstadt, Germany 2 Helmholtz-Institut Mainz, 55099 Mainz, Germany 3 Paul Scherrer Institut, 5232 Villigen PSI, Switzerland 4 University of Bern, Department of Chemistry and Biochemistry, 3012 Bern, Switzerland Abstract. Element 114 was discovered in 2000 by the Dubna-Livermore collaboration, and in 2012 it was named flerovium. It belongs to the group 14 of the periodic table of elements. A strong relativistic stabilisation of 2 2 the valence shell 7s 7p1/2 is expected due to the orbital splitting and the 2 2 contraction not only of the 7s but also of the spherical 7p1/2 closed subshell, resulting in the enhanced volatility and inertness. Flerovium was studied chemically by gas-solid chromatography upon its adsorption on a gold surface. Two experimental results on Fl chemistry have been published so far. Based on observation of three atoms, a weak interaction of flerovium with gold was suggested in the first study. Authors of the second study concluded on the metallic character after the observation of two Fl atoms deposited on gold at room temperature. 1. Introduction Man-made superheavy elements (SHE, atomic number Z ≥ 104) are unique in two aspects. Their nuclei exist only due to nuclear shell effects, and their electron structure is influenced by increasingly important relativistic effects [1–3]. Recently the synthesis of elements 113, 115, 117 and 118 has been approved, thus, the discovery of all elements of the 7th row in the periodic table of elements with proton number Z up to 118 has been acknowledged [4]. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable.