Two-Proton Radioactivity 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
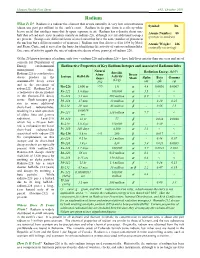
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

Decay Modes of Uranium in the Range 203 <A<299
Indian Journal of Pure & Applied Physics Vol. 58, April 2020, pp. 234-240 Decay modes of Uranium in the range 203 <A<299 H C Manjunathaa*, G R Sridharb,c, P S Damodara Guptab, K N Sridharb, M G Srinivasd & H B Ramalingame aDepartment of Physics, Government College for Women, Kolar 563 101, India bDepartment of Physics, Government First Grade College, Kolar 563 101, India cResearch and Development Centre, Bharathiar University, Coimbatore 641 046, India dDepartment of Physics, Government First Grade College, Mulbagal 563 131, India eDepartment of Physics, Government Arts College, Udumalpet 642 126, India Received 17 February 2020 In the present work, we have considered the total potential as the sum of the coulomb and proximity potential. We have used the recent proximity function to calculate the nuclear potential. The calculated logarithmic half-lives correspond to fission, cluster and alpha decay are compared with that of experiments. We also identified the most probable decay mode by studying branching ratios of these different decay modes. The competition between different decay modes such as fission, cluster radioactivity and alpha decay finds an important role in nuclear structure. Keywords: Nuclear potential, Cluster radioactivity, Alpha decay, Spontaneous fission 1 Introduction Parkhomenko et al.18 studied the alpha decay It is important to study the alpha decay properties properties for odd mass number superheavy nuclei. of superheavy nuclei. Most of the superheavy nuclei Poenaru et al.19 improved the formula for alpha decay are identified through alpha decay process only. halflives around magic numbers by using the SemFIS Many researchers in the nuclear physics field formula. -

Systematical Study of Optical Potential Strengths in Reactions Involving Strongly, Weakly Bound and Exotic Nuclei on 120Sn
Systematical study of optical potential strengths in reactions involving strongly, weakly bound and exotic nuclei on 120Sn M. A. G. Alvarez, J. P. Fern´andez-Garc´ıa, J. L. Le´on-Garc´ıa Departamento FAMN, Universidad de Sevilla, Apartado 1065, 41080 Sevilla, Spain M. Rodr´ıguez-Gallardo Departamento FAMN, Universidad de Sevilla, Apartado 1065, 41080 Sevilla, Spain and Instituto Carlos I de F´ısica Te´orica y Computacional, Universidad de Sevilla, Spain L. R. Gasques, L. C. Chamon, V. A. B. Zagatto, A. L´epine-Szily, J. R. B. Oliveira Universidade de Sao Paulo, Instituto de Fisica, Rua do Matao, 1371, 05508-090, Sao Paulo, SP, Brazil V. Scarduelli Instituto de F´ısica, Universidade Federal Fluminense, 24210-340 Niter´oi,Rio de Janeiro, Brazil and Instituto de F´ısica da Universidade de S~aoPaulo, 05508-090, S~aoPaulo, SP, Brazil B. V. Carlson Departamento de F´ısica, Instituto Tecnol´ogico de Aeron´autica, S~aoJos´edos Campos, SP, Brazil J. Casal Dipartimento di Fisica e Astronomia "G. Galilei" and INFN-Sezione di Padova, Via Marzolo, 8, I-35131, Padova, Italy A. Arazi Laboratorio TANDAR, Comisi´onNacional de Energ´ıa At´omica, Av. Gral. Paz 1499, BKNA1650 San Mart´ın, Argentina D. A. Torres, F. Ramirez Departamento de F´ısica, Universidad Nacional de Colombia, Bogot´a,Colombia (Dated: December 20, 2019) We present new experimental angular distributions for the elastic scattering of 6Li + 120Sn at three bombarding energies. We include these data in a wide systematic involving the elastic scattering of 4;6He, 7Li, 9Be, 10B and 16;18O projectiles on the same target at energies around the respective Coulomb barriers. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Cold Reaction Valleys in the Radioactive Decay of Superheavy 286 112, 292 114 and 296 116 Nuclei
COLD REACTION VALLEYS IN THE RADIOACTIVE DECAY OF SUPERHEAVY 286 112, 292 114 AND 296 116 NUCLEI K. P. Santhosh, S. Sabina School of Pure and Applied Physics, Kannur University, Payyanur Campus, India Cold reaction valleys in the radioactive decay of superheavy nuclei 286 112, 292 114 and 296 116 are studied taking Coulomb and Proximity Potential as the interacting barrier. It is found that in addition to alpha particle, 8Be, 14 C, 28 Mg, 34 Si, 50 Ca, etc. are optimal cases of cluster radioactivity since they lie in the cold valleys. Two other regions of deep minima centered on 208 Pb and 132 Sn are also found. Within our Coulomb and Proximity Potential Model half-life times and other characteristics such as barrier penetrability, decay constant for clusters ranging from alpha particle to 68 Ni are calculated. The computed alpha half-lives match with the values calculated using Viola--Seaborg-- Sobiczewski systematics. The clusters 8Be and 14 C are found to be most probable for 30 emission with T1/2 < 10 s. The alpha-decay chains of the three superheavy nuclei are also studied. The computed alpha decay half-lives are compared with the values predicted by Generalized Liquid Drop Model and they are found to match reasonably well. 1. INTRODUCTION Generally radioactive nuclei decay through alpha and beta decay with subsequent emission of gamma rays in many cases. Again since 1939 it is well known that many radioactive nuclei also decay through spontaneous fission. In 1980 a new type of decay known as cluster radioactivity was predicted by Sandulescu et al. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

2.3 Neutrino-Less Double Electron Capture - Potential Tool to Determine the Majorana Neutrino Mass by Z.Sujkowski, S Wycech
DEPARTMENT OF NUCLEAR SPECTROSCOPY AND TECHNIQUE 39 The above conservatively large systematic hypothesis. TIle quoted uncertainties will be soon uncertainty reflects the fact that we did not finish reduced as our analysis progresses. evaluating the corrections fully in the current analysis We are simultaneously recording a large set of at the time of this writing, a situation that will soon radiative decay events for the processes t e'v y change. This result is to be compared with 1he and pi-+eN v y. The former will be used to extract previous most accurate measurement of McFarlane the ratio FA/Fv of the axial and vector form factors, a et al. (Phys. Rev. D 1984): quantity of great and longstanding interest to low BR = (1.026 ± 0.039)'1 I 0 energy effective QCD theory. Both processes are as well as with the Standard Model (SM) furthermore very sensitive to non- (V-A) admixtures in prediction (Particle Data Group - PDG 2000): the electroweak lagLangian, and thus can reveal BR = (I 038 - 1.041 )*1 0-s (90%C.L.) information on physics beyond the SM. We are currently analyzing these data and expect results soon. (1.005 - 1.008)* 1W') - excl. rad. corr. Tale 1 We see that even working result strongly confirms Current P1IBETA event sxpelilnentstatistics, compared with the the validity of the radiative corrections. Another world data set. interesting comparison is with the prediction based on Decay PIBETA World data set the most accurate evaluation of the CKM matrix n >60k 1.77k element V d based on the CVC hypothesis and ihce >60 1.77_ _ _ results -

Scientific Opportunities with Fast Fragmentation Beams from RIA
Scientific Opportunities with Fast Fragmentation Beams from RIA National Superconducting Cyclotron Laboratory Michigan State University March 2000 EXECUTIVE SUMMARY................................................................................................... 1 1. INTRODUCTION............................................................................................................. 5 2. EXTENDED REACH WITH FAST BEAMS .................................................................. 9 3. SCIENTIFIC MOTIVATION......................................................................................... 12 3.1. Properties of Nuclei far from Stability ..................................................................... 12 3.2. Nuclear Astrophysics................................................................................................ 15 4. EXPERIMENTAL PROGRAM...................................................................................... 20 4.1. Limits of Nuclear Existence ..................................................................................... 20 4.2. Extended and Unusual Distributions of Neutron Matter .......................................... 33 4.3. Properties of Bulk Nuclear Matter............................................................................ 39 4.4. Collective Oscillations.............................................................................................. 51 4.5. Evolution of Nuclear Properties Towards the Drip Lines ........................................ 56 Appendix A: Rate -

Alpha-Decay Chains of Z=122 Superheavy Nuclei Using Cubic Plus Proximity Potential with Improved Transfer Matrix Method
Indian Journal of Pure & Applied Physics Vol. 58, May 2020, pp. 397-403 Alpha-decay chains of Z=122 superheavy nuclei using cubic plus proximity potential with improved transfer matrix method G Naveyaa, S Santhosh Kumarb, S I A Philominrajc & A Stephena* aDepartment of Nuclear Physics, University of Madras, Guindy Campus, Chennai 600 025, India bDepartment of Physics, Kanchi Mamunivar Centre for Post Graduate Studies, Lawspet, Puducherry 605 008, India cDepartment of Physics, Madras Christian College, Chennai 600 059, India Received 4 May 2020 The alpha decay chain properties of Z = 122 isotope in the mass range 298 A 350, even-even nuclei, are studied using a fission-like model with an effective combination of the cubic plus proximity potential in the pre and post-scission regions, wherein the decay rates are calculated using improved transfer matrix method, and the results are in good agreement with other phenomenological formulae such as Universal decay law, Viola-Seaborg, Royer, etc. The nuclear ground-state masses are taken from WS4 mass model. The next minimum in the half-life curves of the decay chain obtained at N=186,178 & 164 suggest the shell closure at N=184, 176 & 162 which coincides well with the predictions of two-centre shell model approach. This study also unveils that the isotopes 298-300, 302, 304-306, 308-310, 312,314122 show 7, 5, 4, 3, 2 and 1 decay chain, respectively. All the other isotopes from A = 316 to 350 may undergo spontaneous fission since the obtained SF half -lives are comparatively less. The predictions in the present study may have an impact in the experimental synthesis and detection of the new isotopes in near future. -

Pair and Single Neutron Transfer with Borromean 8He A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by HAL-CEA Pair and single neutron transfer with Borromean 8He A. Lemasson, A. Navin, M. Rejmund, N. Keeley, V. Zelevinsky, S. Bhattacharyya, A. Shrivastava, Dominique Bazin, D. Beaumel, Y. Blumenfeld, et al. To cite this version: A. Lemasson, A. Navin, M. Rejmund, N. Keeley, V. Zelevinsky, et al.. Pair and single neutron transfer with Borromean 8He. Physics Letters B, Elsevier, 2011, 697, pp.454-458. <10.1016/j.physletb.2011.02.038>. <in2p3-00565792> HAL Id: in2p3-00565792 http://hal.in2p3.fr/in2p3-00565792 Submitted on 14 Feb 2011 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. Pair and single neutron transfer with Borromean 8He A. Lemassona,1, A. Navina,∗, M. Rejmunda, N. Keeleyb, V. Zelevinskyc, S. Bhattacharyyaa,d, A. Shrivastavaa,e, D. Bazinc, D. Beaumelf, Y. Blumenfeldf, A. Chatterjeee, D. Guptaf,2, G. de Francea, B. Jacquota, M. Labicheg, R. Lemmong, V. Nanalh, J. Nybergi, R. G. Pillayh, R. Raabea,3, K. Ramachandrane, J.A. Scarpacif, C. Schmitta, C. Simenelj, I. Stefana,f,4, C.N. -

Problem Set 3 Solutions
22.01 Fall 2016, Problem Set 3 Solutions October 9, 2016 Complete all the assigned problems, and do make sure to show your intermediate work. 1 Activity and Half Lives 1. Given the half lives and modern-day abundances of the three natural isotopes of uranium, calculate the isotopic fractions of uranium when the Earth first formed 4.5 billion years ago. Today, uranium consists of 0.72% 235U, 99.2745% 238U, and 0.0055% 234U. However, it is clear that the half life of 234U (245,500 years) is so short compared to the lifetime of the Earth (4,500,000,000 years) that it would have all decayed away had there been some during the birth of the Earth. Therefore, we look a little closer, and find that 234U is an indirect decay product of 238U, by tracing it back from its parent nuclides on the KAERI table: α β− β− 238U −! 234T h −! 234P a −! 234U (1) Therefore we won’t consider there being any more 234U than would normally be in equi librium with the 238U around at the time. We set up the two remaining equations as follows: −t t ;235 −t t ;238 = 1=2 = 1=2 N235 = N0235 e N238 = N0238 e (2) Using the current isotopic abundances from above as N235 and N238 , the half lives from n 9 t 1 1 the KAERI Table of Nuclides t =2;235 = 703800000 y; t =2;238 = 4:468 · 10 y , and the lifetime of the earth in years (keeping everything in the same units), we arrive at the following expressions for N0235 and N0238 : N235 0:0072 N238 0:992745 N0235 =−t = 9 = 4:307N0238 =−t = 9 = 2:718 (3) =t1 ;235 −4:5·10 =7:038·108 =t1 ;238 −4:5·10 =4:468·109 e =2 e e =2 e Finally, taking the ratios of these two relative abundances gives us absolute abundances: 4:307 2:718 f235 = = 0:613 f238 = = 0:387 (4) 4:307 + 2:718 4:307 + 2:718 235U was 61.3% abundant, and 238U was 38.7% abundant. -
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit .