Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

22Sra 226Ra and 222Rn in ' SOUTH CAROLINA GROUND WATER
Report No. 95 WRRI 22sRa 226Ra AND 222Rn IN ' SOUTH CAROLINA GROUND WATER: MEASUR~MEN-T TECHNIQUES AND ISOTOPE RELATIONSHIPS GlANNll\li FCUN~On o;: t,GR!CUL.TU~~tcoNOMlC2' ~~J.i\2Y J\li·iU ('lJ ~C 4· 1982 WATER RESOURCES RESEARCH INSTITUTE CLEMSON UNIVERSITY Clemson, .South Carolina JANUARY 1982 Water Resources Research Institute Clemson University Clemson, South Carolina 29631 ! 228Ra, 226Ra AND 222 Rn IN SOUTH CAROLINA GROUND WATER: MEASUREMENT TECHNIQUES AND ISOTOPE RELATIONSHIPS by Jacqueline Michel, Philip T. King and Willard S. Moore Department of Geology University of South Carolina Columbia, South Carolina 29208 The work upon which this puhlication is based was supported in part by funds provided by the Office of Water Research and Technology, project !!' No. OWRT-B-127-SC, U.S. Department of the Interior, fvashington, D. C., as authorized by the Water Research and Development Act of 1978 (PL95-467). r Project agreement No. 14-34-0001-9158 Period of Investigation: October 1979 - September 1980 Clemson University Water Resources Research Institute Technical Report No. 95 Contents of this publication do not necessarily reflect the views and policies of the Office of Water Research and Technology, U.S. Department of Interior, nor does mention of trade names or cormnercial products con stitute their endorsement or recommendation for use by the U.S. Goverrtment. ACKNOWLEDGEMENTS Much appreciation goes to Lewis Shaw, Rossie Stephens, Jim Ferguson and a large number of district personnel at the South Carolina Department of Health and Environmental Control who assisted us in field collection and provided much helpful information. Dr. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Experimental Γ Ray Spectroscopy and Investigations of Environmental Radioactivity
Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity BY RANDOLPH S. PETERSON 216 α Po 84 10.64h. 212 Pb 1- 415 82 0- 239 β- 01- 0 60.6m 212 1+ 1630 Bi 2+ 1513 83 α β- 2+ 787 304ns 0+ 0 212 α Po 84 Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity Randolph S. Peterson Physics Department The University of the South Sewanee, Tennessee Published by Spectrum Techniques All Rights Reserved Copyright 1996 TABLE OF CONTENTS Page Introduction ....................................................................................................................4 Basic Gamma Spectroscopy 1. Energy Calibration ................................................................................................... 7 2. Gamma Spectra from Common Commercial Sources ........................................ 10 3. Detector Energy Resolution .................................................................................. 12 Interaction of Radiation with Matter 4. Compton Scattering............................................................................................... 14 5. Pair Production and Annihilation ........................................................................ 17 6. Absorption of Gammas by Materials ..................................................................... 19 7. X Rays ..................................................................................................................... 21 Radioactive Decay 8. Multichannel Scaling and Half-life ..................................................................... -

Two-Proton Radioactivity 2
Two-proton radioactivity Bertram Blank ‡ and Marek P loszajczak † ‡ Centre d’Etudes Nucl´eaires de Bordeaux-Gradignan - Universit´eBordeaux I - CNRS/IN2P3, Chemin du Solarium, B.P. 120, 33175 Gradignan Cedex, France † Grand Acc´el´erateur National d’Ions Lourds (GANIL), CEA/DSM-CNRS/IN2P3, BP 55027, 14076 Caen Cedex 05, France Abstract. In the first part of this review, experimental results which lead to the discovery of two-proton radioactivity are examined. Beyond two-proton emission from nuclear ground states, we also discuss experimental studies of two-proton emission from excited states populated either by nuclear β decay or by inelastic reactions. In the second part, we review the modern theory of two-proton radioactivity. An outlook to future experimental studies and theoretical developments will conclude this review. PACS numbers: 23.50.+z, 21.10.Tg, 21.60.-n, 24.10.-i Submitted to: Rep. Prog. Phys. Version: 17 December 2013 arXiv:0709.3797v2 [nucl-ex] 23 Apr 2008 Two-proton radioactivity 2 1. Introduction Atomic nuclei are made of two distinct particles, the protons and the neutrons. These nucleons constitute more than 99.95% of the mass of an atom. In order to form a stable atomic nucleus, a subtle equilibrium between the number of protons and neutrons has to be respected. This condition is fulfilled for 259 different combinations of protons and neutrons. These nuclei can be found on Earth. In addition, 26 nuclei form a quasi stable configuration, i.e. they decay with a half-life comparable or longer than the age of the Earth and are therefore still present on Earth. -

Analysis of Isotopes of Radium, Uranium and Thorium. 210Pb-210Po
Analysis of isotopes of radium, uranium and thorium. 210Pb-210Po Per Roos Risø National Laboratory for Sustainable Energy, Technical university of Denmark Radium isotopes Adsorption of Ra on MnO2 & direct alpha spectrometry Coating of polyamid (Nylon) with MnO2 (KMnO4 solution) ……half-life of Ra-224 is 88h Very thin MnO2 layer, poor yield but excellent resolution Electrodeposited sources Direct 20 days 6 months Analysis of 226Ra in water • Weigh up a suitable amount of water (10 litre is mostly sufficient). • Add HNO3 to pH 1-2. • Add 133Ba tracer (ca 10 Bq). • Add about 13 ml of a 0.2M KMnO4. • Adjust pH to about 8-9 with ammonia. • Add 15 ml 0.3M MnCl2 and stir for some 5 minutes. • Adjust with KMnO4 or MnCl2 to make the sample water phase slightly pink. • Collect the precipitate and centrifuge • Dissolve precipitate in 1M HCl-1%H2O2. • Add about 2g K2SO4+1ml H2SO4 and dilute to 100 ml with water. • Heat the sample to dissolve the K2SO4 completely. • Add 30 mg Pb2+. Heat for 20 minutes and stir. • Centrifuge the precipitate and discard supernate. • Wash precipitate with 100 ml of a solution of 0.2M H2SO4 and 0.1 M K2SO4 • Centrifuge the precipitate and discard supernate • Dissolve the PbSO4-precipitate with 3ml 0.1M EDTA at pH 10. • Transfer the solution to the scintillation vial (low diffusion PE-vial). • Wash centrifuge tube with 2 ml 0.1M EDTA and transfer to Scintillation vial. • Add 10ml OptiFluor 0 and fill with deionised water to total 20 ml. • Allow for ingrowth of 222Rn during 3 weeks and measure by LSQ. -

Naturally Occurring Radioactive Material (NORM VI) (NORM Material Radioactive Occurring Naturally Naturally Occurring Radioactive Material
Naturally Occurring Radioactive Material (NORM VI) Naturally Occurring Radioactive Material All minerals and raw materials contain radionuclides of natural origin. For (NORM VI) most human activities involving minerals and raw materials, the levels of exposure to these radionuclides are not significantly greater than normal background levels. However, certain work activities can give rise to signif- Proceedings icantly enhanced exposures that may need to be controlled by regulation. Material giving rise to these enhanced exposures has become known as of an International Symposium naturally occurring radioactive material (NORM). Historically, most regula- tory attention has been focused on the mining and processing of uranium Marrakesh, Morocco, 22–26 March 2010 ore, because such activities are a direct consequence of the radioactivity in the ore and form part of the nuclear fuel cycle. Over the past decade or two, however, more and more countries have introduced measures to reg- 1 ulate exposures arising from a wider range of natural sources. The NORM VI symposium, which was held in Marrakesh, Morocco, from 22–26 March 2010, provided an important opportunity to review the developments that had taken place during the three year period since the NORM V sym- posium in 2007. This period, which began with the publication of new radiation protection recommendations by the International Commission on Radiological Protection, was characterized by ongoing activities to revise international standards on radiation protection and safety. These Proceedings contain all 38 papers accepted for oral presentation and four rapporteur reports, as well as a summary that concludes with the main findings of the symposium. Text versions of 43 poster presentations are provided on a CD-ROM which accompanies these Proceedings. -

Radionuclides Notice of Data Availability Technical Support Document
Radionuclides Notice of Data Availability Technical Support Document March 2000 Targeting and Analysis Branch Standards and Risk Management Division Office of Ground Water and Drinking Water United States Environmental Protection Agency Prepared by: USEPA Office of Ground Water and Drinking Water in collaboration with USEPA Office of Indoor Air and Radiation United States Geological Survey Preface Under the authority of the Safe Drinking Water Act (SDWA) and its amendments, EPA sets drinking water standards for contaminants, which potentially pose a threat to public health via a public drinking water source. In 1976, EPA promulgated National Interim Primary Drinking Water Regulations (NIPDWR) for three categories of radionuclides: gross alpha emitters, radium 226 and 228 combined, and gross beta and photon emitters. The 1976 NIPDWRs for these radionuclides regulated: 1. gross alpha at 15 picoCuries per liter (pCi/L)(excluding radon and uranium); 2. radium-226 and radium-228 combined at 5 pCi/L; 3. and beta/photon emitters at a 4 millirem dose of radioactivity. The 1986 reauthorization of the Safe Drinking Water Act (SDWA) declared the interim standards for these radionuclides to be final National Primary Drinking Water Regulations (NPDWR). The 1986 amendment also required standards to be set as close to the maximum contaminant level goal (MCLG; the health goal) as possible. At that time, radionuclides did not have an MCLG. In 1991 (56 FR 33050), EPA proposed: < A Maximum Contaminant Level Goal (MCLG) of zero for all radionuclides; < A -

"Indoor Radon and Radon Decay Product Measurement Device Protocols"
"Indoor Radon and Radon Decay Product Measurement Device Protocols" U.S. Environmental Protection Agency Office of Air and Radiation (6604J) EPA 402-R-92-004, July 1992 (revised) Table of Contents Disclaimer Acknowledgements Significant Changes in This Revision Radon and Radon Decay Product Measurement Methods Section 1: General Considerations 1.1 Introduction and Background 1.2 General Guidance on Measurement Strategy, Measurement Conditions, Device Location Selection, and Documentation 1.3 Quality Assurance Section 2: Indoor Radon Measurement Device Protocols 2.1 Protocol for Using Continuous Radon Monitors (CR) to Measure Indoor Radon Concentrations 2.2 Protocol for Using Alpha Track Detectors (AT or ATD) to Measure Indoor Radon Concentrations 2.4 Protocol for Using Activated Charcoal Adsorption Devices (AC) to Measure Indoor Radon Concentrations 2.5 Protocol for Using Charcoal Liquid Scintillation (LS) Devices to Measure Indoor Radon Concentrations 2.6 Protocol for Using Grab Radon Sampling (GB, GC, GS), Pump/Collapsible Bag Devices (PB), and Three-Day Integrating Evacuated Scintillation Cells (SC) to Measure Indoor Radon Concentrations 2.7 Interim Protocol for Using Unfiltered Track Detectors (UT) to Measure Indoor Radon Concentrations Section 3: Indoor Radon Decay Product Measurement Device Protocols 3.1 Protocol for Using Continuous Working Level Monitors (CW) to Measure Indoor Radon Decay Product Concentrations 3.2 Protocol for Using Radon Progeny Integrating Sampling Units (RPISU or RP) to Measure Indoor Radon Decay Product Concentrations 3.3 Protocol for Using Grab Sampling-Working Level (GW) to Measure Indoor Radon Decay Product Concentrations Glossary References Please Note: EPA closed its National Radon Proficiency Program (RPP) in 1998. -

Energy What Is a Nuclear Reaction?
4/16/2016 Option C: Energy C.3 : Nuclear Fusion and Fission What is a nuclear reaction? • A nuclear reaction is any reaction that involves the nucleus. • These reactions change the identity of an atom, as opposed to chemical reactions which only involve valence electrons. 1 4/16/2016 The nucleus • The nucleus is made up of protons and neutrons. • We know what the protons do – they provide an electrostatic attraction to the electrons close… but what about the neutrons? The Neutrons • The major function of the neutrons is to hold the nucleus together. • The neutrons provide a strong nuclear force of attraction within the nucleus, counteracting the repulsion between the positively charged protons. 2 4/16/2016 How is the nucleus held together? • In the 1930’s it was first observed that the mass of an atoms nucleus is less than the sum of the masses of the protons + neutrons…? • Some of the mass of the nucleus is converted into energy to hold the nucleus together. 3 4/16/2016 Mass Defect • The difference in mass of the nucleus and it’s parts is referred to as the mass defect, and the energy (e=mc 2) it provided is called the nuclear binding energy. 4 4/16/2016 Nuclear vs. Chemical Reactions • This nuclear binding energy is released during nuclear reactions (fission & fusion), and is ~1,000,000X greater than the chemical bond energy released during chemical reactions. What makes an isotope radioactive? • Elements are radioactive when their nucleus is unstable. • The stabilizing force of the neutrons is effective for smaller elements, though all elements above lead are radioactive. -

Nuclear Data As the Alternative Information of Decay and Analyzer
Usman Kadir, NuCLEAR Data as The alternative Information of decay and Analyzer NuCLEAR Data as The Alternative Information of Decay and Analyzer Usman Kadir Pusat Pendidikan dan Pelatihan BA TAN Abstract NuCLEAR Data is the name of visual, color-coded computer program of "Chart of Nuclides" or "Segre Chart". The program gives you comprehensive nuclear decay data that include data on alpha, beta, gamma, x-ray decay etc., and comprehensive search by energy, intensity, half life, radiation type or name of nuclide. NuCLEAR Data is able to show visual decay product and couldfunction as Multi Nuclides Identification and Multi Elements Identification Analysis. It is constructed using Visual Basic Compiler. Abstak NuCLEAR Data adalah nama program komputer untuk peta nuklida atau Segre Chart; data tervisual dan dikodekan warna. Program ini memberikan anda data peluruhan nuklir secara komprehensif, yang meliputi peluruhan alpha, beta, gamma, sinar-x dan dapat mencari energi, intensitas, waktu paro, jenis radiasi dan nama nuklida. NuCLEAR Data dapat menampilkan produk peluruhan secara visual dan dapat berfungsi sebagai analisis untuk Identifikasi multi nuklida dan identifikasi unsur-unsur. Program ini dibuat dengan Visual Basic. Chart of the Nuclides The "Chart of the Nuclides" or Segre Chart is shown in a colored mode, including details Chart of the Nuclides is a plot of all known for each nuclide's meta-stable states. nuclides as a function of Z (Atomic number), in the y-axis, and N (number of neutrons), in NuCLEAR for Windows 95/98/2000IXP is a the x-axis. visual Nuclear Data Library. It can be used as a standalone information system. -

Occurrence of Radium-224, Radium
Prepared in cooperation with the New Jersey Department of Environmental Protection Occurrence of Radium-224, Radium-226 and Radium-228 in Water from the Vincentown and Wenonah-Mount Laurel Aquifers, the Englishtown Aquifer System, and the Hornerstown and Red Bank Sands, Southwestern and South-Central New Jersey Scientific Investigations Report 2007-5064 U.S. Department of the Interior U.S. Geological Survey Version 1.1 Occurrence of Radium-224, Radium-226 and Radium-228 in Water from the Vincentown and Wenonah-Mount Laurel Aquifers, the Englishtown Aquifer System, and the Hornerstown and Red Bank Sands, Southwestern and South-Central New Jersey By Vincent T. dePaul and Zoltan Szabo Prepared in cooperation with the New Jersey Department of Environmental Protection Scientific Investigations Report 2007–5064 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2007 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS--the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. -
Modern Physics to Which He Did This Observation, Usually to Within About 0.1%
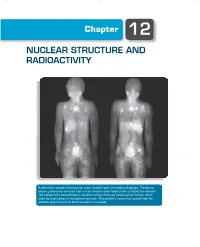
Chapter 12 NUCLEAR STRUCTURE AND RADIOACTIVITY Radioactive isotopes have proven to be valuable tools for medical diagnosis. The photo shows gamma-ray emission from a man who has been treated with a radioactive element. The radioactivity concentrates in locations where there are active cancer tumors, which show as bright areas in the gamma-ray scan. This patient’s cancer has spread from his prostate gland to several other locations in his body. 370 Chapter 12 | Nuclear Structure and Radioactivity The nucleus lies at the center of the atom, occupying only 10−15 of its volume but providing the electrical force that holds the atom together. Within the nucleus there are Z positive charges. To keep these charges from flying apart, the nuclear force must supply an attraction that overcomes their electrical repulsion. This nuclear force is the strongest of the known forces; it provides nuclear binding energies that are millions of times stronger than atomic binding energies. There are many similarities between atomic structure and nuclear structure, which will make our study of the properties of the nucleus somewhat easier. Nuclei are subject to the laws of quantum physics. They have ground and excited states and emit photons in transitions between the excited states. Just like atomic states, nuclear states can be labeled by their angular momentum. There are, however, two major differences between the study of atomic and nuclear properties. In atomic physics, the electrons experience the force provided by an external agent, the nucleus; in nuclear physics, there is no such external agent. In contrast to atomic physics, in which we can often consider the interactions among the electrons as a perturbation to the primary interaction between electrons and nucleus, in nuclear physics the mutual interaction of the nuclear constituents is just what provides the nuclear force, so we cannot treat this complicated many- body problem as a correction to a single-body problem.