Radioactivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -
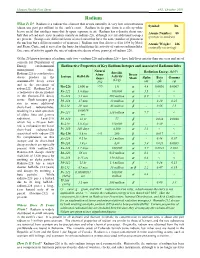
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

Qcd in Heavy Quark Production and Decay
QCD IN HEAVY QUARK PRODUCTION AND DECAY Jim Wiss* University of Illinois Urbana, IL 61801 ABSTRACT I discuss how QCD is used to understand the physics of heavy quark production and decay dynamics. My discussion of production dynam- ics primarily concentrates on charm photoproduction data which is compared to perturbative QCD calculations which incorporate frag- mentation effects. We begin our discussion of heavy quark decay by reviewing data on charm and beauty lifetimes. Present data on fully leptonic and semileptonic charm decay is then reviewed. Mea- surements of the hadronic weak current form factors are compared to the nonperturbative QCD-based predictions of Lattice Gauge The- ories. We next discuss polarization phenomena present in charmed baryon decay. Heavy Quark Effective Theory predicts that the daugh- ter baryon will recoil from the charmed parent with nearly 100% left- handed polarization, which is in excellent agreement with present data. We conclude by discussing nonleptonic charm decay which are tradi- tionally analyzed in a factorization framework applicable to two-body and quasi-two-body nonleptonic decays. This discussion emphasizes the important role of final state interactions in influencing both the observed decay width of various two-body final states as well as mod- ifying the interference between Interfering resonance channels which contribute to specific multibody decays. "Supported by DOE Contract DE-FG0201ER40677. © 1996 by Jim Wiss. -251- 1 Introduction the direction of fixed-target experiments. Perhaps they serve as a sort of swan song since the future of fixed-target charm experiments in the United States is A vast amount of important data on heavy quark production and decay exists for very short. -

What Is the Nature of Neutrinos?
16th Neutrino Platform Week 2019: Hot Topics in Neutrino Physics CERN, Switzerland, Switzerland, 7– 11 October 2019 Matrix Elements for Neutrinoless Double Beta Decay Fedor Šimkovic OUTLINE I. Introduction (Majorana ν’s) II. The 0νββ-decay scenarios due neutrinos exchange (simpliest, sterile ν, LR-symmetric model) III. DBD NMEs – Current status (deformation, scaling relation?, exp. support, ab initio… ) IV. Quenching of gA (Ikeda sum rule, 2νββ-calc., novel approach for effective gA ) V. Looking for a signal of lepton number violation (LHC study, resonant 0νECEC …) Acknowledgements: A. Faessler (Tuebingen), P. Vogel (Caltech), S. Kovalenko (Valparaiso U.), M. Krivoruchenko (ITEP Moscow), D. Štefánik, R. Dvornický (Comenius10/8/2019 U.), A. Babič, A. SmetanaFedor(IEAP SimkovicCTU Prague), … 2 After 89/63 years Fundamental ν properties No answer yet we know • Are ν Dirac or • 3 families of light Majorana? (V-A) neutrinos: •Is there a CP violation ν , ν , ν ν e µ τ e in ν sector? • ν are massive: • Are neutrinos stable? we know mass • What is the magnetic squared differences moment of ν? • relation between • Sterile neutrinos? flavor states • Statistical properties and mass states ν µ of ν? Fermionic or (neutrino mixing) partly bosonic? Currently main issue Nature, Mass hierarchy, CP-properties, sterile ν The observation of neutrino oscillations has opened a new excited era in neutrino physics and represents a big step forward in our knowledge of neutrino10/8/2019 properties Fedor Simkovic 3 Symmetric Theory of Electron and Positron Nuovo Cim. 14 (1937) 171 CNNP 2018, Catania, October 15-21, 2018 10/8/2019 Fedor Simkovic 4 ν ↔ ν- oscillation (neutrinos are Majorana particles) 1968 Gribov, Pontecorvo [PLB 28(1969) 493] oscillations of neutrinos - a solution of deficit10/8/2019 of solar neutrinos in HomestakeFedor Simkovic exp. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Discovery of the Isotopes with Z<= 10
Discovery of the Isotopes with Z ≤ 10 M. Thoennessen∗ National Superconducting Cyclotron Laboratory and Department of Physics and Astronomy, Michigan State University, East Lansing, MI 48824, USA Abstract A total of 126 isotopes with Z ≤ 10 have been identified to date. The discovery of these isotopes which includes the observation of unbound nuclei, is discussed. For each isotope a brief summary of the first refereed publication, including the production and identification method, is presented. arXiv:1009.2737v1 [nucl-ex] 14 Sep 2010 ∗Corresponding author. Email address: [email protected] (M. Thoennessen) Preprint submitted to Atomic Data and Nuclear Data Tables May 29, 2018 Contents 1. Introduction . 2 2. Discovery of Isotopes with Z ≤ 10........................................................................ 2 2.1. Z=0 ........................................................................................... 3 2.2. Hydrogen . 5 2.3. Helium .......................................................................................... 7 2.4. Lithium ......................................................................................... 9 2.5. Beryllium . 11 2.6. Boron ........................................................................................... 13 2.7. Carbon.......................................................................................... 15 2.8. Nitrogen . 18 2.9. Oxygen.......................................................................................... 21 2.10. Fluorine . 24 2.11. Neon........................................................................................... -

Ivan V. Ani~In Faculty of Physics, University of Belgrade, Belgrade, Serbia and Montenegro
THE NEUTRINO Its past, present and future Ivan V. Ani~in Faculty of Physics, University of Belgrade, Belgrade, Serbia and Montenegro The review consists of two parts. In the first part the critical points in the past, present and future of neutrino physics (nuclear, particle and astroparticle) are briefly reviewed. In the second part the contributions of Yugoslav physics to the physics of the neutrino are commented upon. The review is meant as a first reading for the newcomers to the field of neutrino physics. Table of contents Introduction A. GENERAL REVIEW OF NEUTRINO a.2. Electromagnetic properties of the neutrino PHYSICS b. Neutrino in branches of knowledge other A.1. Short history of the neutrino than neutrino physics A.1.1. First epoch: 1930-1956 A.2. The present status of the neutrino A.1.2. Second epoch: 1956-1958 A.3. The future of neutrino physics A.1.3. Third epoch: 1958-1983 A.1.4. Fourth epoch: 1983-2001 B. THE YUGOSLAV CONNECTION a. The properties of the neutrino B.1. The Thallium solar neutrino experiment a.1. Neutrino masses B.2. The neutrinoless double beta decay a.1.1. Direct methods Epilogue a.1.2. Indirect methods References a.1.2.1. Neutrinoless double beta decay a.1.2.2. Neutrino oscillations 1 Introduction The neutrinos appear to constitute by number of species not less than one quarter of the particles which make the world, and even half of the stable ones. By number of particles in the Universe they are perhaps second only to photons. -

A Measurement of the 2 Neutrino Double Beta Decay Rate of 130Te in the CUORICINO Experiment by Laura Katherine Kogler
A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Physics in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Stuart J. Freedman, Chair Professor Yury G. Kolomensky Professor Eric B. Norman Fall 2011 A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment Copyright 2011 by Laura Katherine Kogler 1 Abstract A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler Doctor of Philosophy in Physics University of California, Berkeley Professor Stuart J. Freedman, Chair CUORICINO was a cryogenic bolometer experiment designed to search for neutrinoless double beta decay and other rare processes, including double beta decay with two neutrinos (2νββ). The experiment was located at Laboratori Nazionali del Gran Sasso and ran for a period of about 5 years, from 2003 to 2008. The detector consisted of an array of 62 TeO2 crystals arranged in a tower and operated at a temperature of ∼10 mK. Events depositing energy in the detectors, such as radioactive decays or impinging particles, produced thermal pulses in the crystals which were read out using sensitive thermistors. The experiment included 4 enriched crystals, 2 enriched with 130Te and 2 with 128Te, in order to aid in the measurement of the 2νββ rate. The enriched crystals contained a total of ∼350 g 130Te. The 128-enriched (130-depleted) crystals were used as background monitors, so that the shared backgrounds could be subtracted from the energy spectrum of the 130- enriched crystals. -

Photofission Cross Sections of 238U and 235U from 5.0 Mev to 8.0 Mev Robert Andrew Anderl Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1972 Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV Robert Andrew Anderl Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Nuclear Commons, and the Oil, Gas, and Energy Commons Recommended Citation Anderl, Robert Andrew, "Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV " (1972). Retrospective Theses and Dissertations. 4715. https://lib.dr.iastate.edu/rtd/4715 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction, 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity, 2. -

Mean Lifetime Part 3: Cosmic Muons
MEAN LIFETIME PART 3: MINERVA TEACHER NOTES DESCRIPTION Physics students often have experience with the concept of half-life from lessons on nuclear decay. Teachers may introduce the concept using M&M candies as the decaying object. Therefore, when students begin their study of decaying fundamental particles, their understanding of half-life may be at the novice level. The introduction of mean lifetime as used by particle physicists can cause confusion over the difference between half-life and mean lifetime. Students using this activity will develop an understanding of the difference between half-life and mean lifetime and the reason particle physicists prefer mean lifetime. Mean Lifetime Part 3: MINERvA builds on the Mean Lifetime Part 1: Dice which uses dice as a model for decaying particles, and Mean Lifetime Part 2: Cosmic Muons which uses muon data collected with a QuarkNet cosmic ray muon detector (detector); however, these activities are not required prerequisites. In this activity, students access authentic muon data collected by the Fermilab MINERvA detector in order to determine the half-life and mean lifetime of these fundamental particles. This activity is based on the Particle Decay activity from Neutrinos in the Classroom (http://neutrino-classroom.org/particle_decay.html). STANDARDS ADDRESSED Next Generation Science Standards Science and Engineering Practices 4. Analyzing and interpreting data 5. Using mathematics and computational thinking Crosscutting Concepts 1. Patterns 2. Cause and Effect: Mechanism and Explanation 3. Scale, Proportion, and Quantity 4. Systems and System Models 7. Stability and Change Common Core Literacy Standards Reading 9-12.7 Translate quantitative or technical information . -
R-Process: Observations, Theory, Experiment
r-process: observations, theory, experiment H. Schatz Michigan State University National Superconducting Cyclotron Laboratory Joint Institute for Nuclear Astrophysics 1. Observations: do we need s,r,p-process and LEPP? 2. r-process (and LEPP?) models 3. r-process experiments SNR 0103-72.6 Credit: NASA/CXC/PSU/S.Park et al. Origin of the heavy elements in the solar system s-process: secondary • nuclei can be studied Æ reliable calculations • site identified • understood? Not quite … r-process: primary • most nuclei out of reach • site unknown p-process: secondary (except for νp-process) Æ Look for metal poor`stars (Pagel, Fig 6.8) To learn about the r-process Heavy elements in Metal Poor Halo Stars CS22892-052 (Sneden et al. 2003, Cowan) 2 1 + solar r CS 22892-052 ) H / X CS22892-052 ( g o red (K) giant oldl stars - formed before e located in halo Galaxyc was mixed n distance: 4.7 kpc theya preserve local d mass ~0.8 M_sol n pollutionu from individual b [Fe/H]= −3.0 nucleosynthesisa events [Dy/Fe]= +1.7 recall: element number[X/Y]=log(X/Y)-log(X/Y)solar What does it mean: for heavy r-process? For light r-process? • stellar abundances show r-process • process is not universal • process is universal • or second process exists (not visible in this star) Conclusions depend on s-process Look at residuals: Star – solar r Solar – s-process – p-process s-processSimmerer from Simmerer (Cowan et etal.) al. /Lodders (Cowan et al.) s-processTravaglio/Lodders from Travaglio et al. -0.50 -0.50 -1.00 -1.00 -1.50 -1.50 log e log e -2.00 -2.00 -2.50 -2.50 30 40 50 60 70 80 90 30 40 50 60 70 80 90 Element number Element number ÆÆNeedNeed reliable reliable s-process s-process (models (models and and nu nuclearclear data, data, incl. -

Electron Capture in Stars
Electron capture in stars K Langanke1;2, G Mart´ınez-Pinedo1;2;3 and R.G.T. Zegers4;5;6 1GSI Helmholtzzentrum f¨urSchwerionenforschung, D-64291 Darmstadt, Germany 2Institut f¨urKernphysik (Theoriezentrum), Department of Physics, Technische Universit¨atDarmstadt, D-64298 Darmstadt, Germany 3Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨ur Schwerionenforschung, D-64291 Darmstadt, Germany 4 National Superconducting Cyclotron Laboratory, Michigan State University, East Lansing, Michigan 48824, USA 5 Joint Institute for Nuclear Astrophysics: Center for the Evolution of the Elements, Michigan State University, East Lansing, Michigan 48824, USA 6 Department of Physics and Astronomy, Michigan State University, East Lansing, Michigan 48824, USA E-mail: [email protected], [email protected], [email protected] Abstract. Electron captures on nuclei play an essential role for the dynamics of several astrophysical objects, including core-collapse and thermonuclear supernovae, the crust of accreting neutron stars in binary systems and the final core evolution of intermediate mass stars. In these astrophysical objects, the capture occurs at finite temperatures and at densities at which the electrons form a degenerate relativistic electron gas. The capture rates can be derived in perturbation theory where allowed nuclear transitions (Gamow-Teller transitions) dominate, except at the higher temperatures achieved in core-collapse supernovae where also forbidden transitions contribute significantly to the rates. There has been decisive progress in recent years in measuring Gamow-Teller (GT) strength distributions using novel experimental techniques based on charge-exchange reactions. These measurements provide not only data for the GT distributions of ground states for many relevant nuclei, but also serve as valuable constraints for nuclear models which are needed to derive the capture rates for the arXiv:2009.01750v1 [nucl-th] 3 Sep 2020 many nuclei, for which no data exist yet.