Uses of Isotopic Neutron Sources in Elemental Analysis Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MASTER 9700 South Cass Avenue Argonne, Illinois 60439 USA
ANL/KDH—SO DE84 001440 ANL/NDM-80 NEUTRON TOTAL CROSS SECTION MEASUREMENTS IN THE ENERGY REGION FROM 47 key to 20 MeV* by W. P. Poenitz and J. F. Whalon Applied Physics Division May, 1983 *This work supported by the U.S. Department of Energy Argonne National Laboratory MASTER 9700 South Cass Avenue Argonne, Illinois 60439 USA fflSTRtBtfUOU OF miS DOCUMENT IS UNLIMITED NUCLEAR DATA AMD MEASUREMENTS SERIES The Nuclear Data and Measurements Series presents results of studies in the field of microscopic nuclear data. The primary objective is the dissemination of information in the comprehensive form required for nuclear technology applications. This Series is devoted to: a) measured microscopic nuclear parameters, b) experimental techniques and facilities employed in measurements, c) the analysis, correlation and interpretation of nuclear data, and d) the evaluation of nuclear data. Contributions to this Series are reviewed to assure technical competence and, unless otherwise stated, the contents can be formally referenced. This Series does not supplant formal journal publication but it does provide the more extensive informa- tion required for technological applications (e.g., tabulated numerical data) in a timely manner. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or retpoosi- bility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Refer- ence herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recom- mendation, or favoring by the United States Government or any agency thereof. -

Discovery of the Isotopes with Z<= 10
Discovery of the Isotopes with Z ≤ 10 M. Thoennessen∗ National Superconducting Cyclotron Laboratory and Department of Physics and Astronomy, Michigan State University, East Lansing, MI 48824, USA Abstract A total of 126 isotopes with Z ≤ 10 have been identified to date. The discovery of these isotopes which includes the observation of unbound nuclei, is discussed. For each isotope a brief summary of the first refereed publication, including the production and identification method, is presented. arXiv:1009.2737v1 [nucl-ex] 14 Sep 2010 ∗Corresponding author. Email address: [email protected] (M. Thoennessen) Preprint submitted to Atomic Data and Nuclear Data Tables May 29, 2018 Contents 1. Introduction . 2 2. Discovery of Isotopes with Z ≤ 10........................................................................ 2 2.1. Z=0 ........................................................................................... 3 2.2. Hydrogen . 5 2.3. Helium .......................................................................................... 7 2.4. Lithium ......................................................................................... 9 2.5. Beryllium . 11 2.6. Boron ........................................................................................... 13 2.7. Carbon.......................................................................................... 15 2.8. Nitrogen . 18 2.9. Oxygen.......................................................................................... 21 2.10. Fluorine . 24 2.11. Neon........................................................................................... -

Photofission Cross Sections of 238U and 235U from 5.0 Mev to 8.0 Mev Robert Andrew Anderl Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1972 Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV Robert Andrew Anderl Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Nuclear Commons, and the Oil, Gas, and Energy Commons Recommended Citation Anderl, Robert Andrew, "Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV " (1972). Retrospective Theses and Dissertations. 4715. https://lib.dr.iastate.edu/rtd/4715 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction, 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity, 2. -
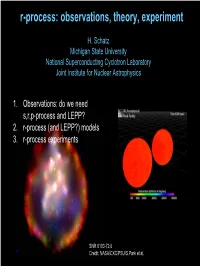
R-Process: Observations, Theory, Experiment
r-process: observations, theory, experiment H. Schatz Michigan State University National Superconducting Cyclotron Laboratory Joint Institute for Nuclear Astrophysics 1. Observations: do we need s,r,p-process and LEPP? 2. r-process (and LEPP?) models 3. r-process experiments SNR 0103-72.6 Credit: NASA/CXC/PSU/S.Park et al. Origin of the heavy elements in the solar system s-process: secondary • nuclei can be studied Æ reliable calculations • site identified • understood? Not quite … r-process: primary • most nuclei out of reach • site unknown p-process: secondary (except for νp-process) Æ Look for metal poor`stars (Pagel, Fig 6.8) To learn about the r-process Heavy elements in Metal Poor Halo Stars CS22892-052 (Sneden et al. 2003, Cowan) 2 1 + solar r CS 22892-052 ) H / X CS22892-052 ( g o red (K) giant oldl stars - formed before e located in halo Galaxyc was mixed n distance: 4.7 kpc theya preserve local d mass ~0.8 M_sol n pollutionu from individual b [Fe/H]= −3.0 nucleosynthesisa events [Dy/Fe]= +1.7 recall: element number[X/Y]=log(X/Y)-log(X/Y)solar What does it mean: for heavy r-process? For light r-process? • stellar abundances show r-process • process is not universal • process is universal • or second process exists (not visible in this star) Conclusions depend on s-process Look at residuals: Star – solar r Solar – s-process – p-process s-processSimmerer from Simmerer (Cowan et etal.) al. /Lodders (Cowan et al.) s-processTravaglio/Lodders from Travaglio et al. -0.50 -0.50 -1.00 -1.00 -1.50 -1.50 log e log e -2.00 -2.00 -2.50 -2.50 30 40 50 60 70 80 90 30 40 50 60 70 80 90 Element number Element number ÆÆNeedNeed reliable reliable s-process s-process (models (models and and nu nuclearclear data, data, incl. -

HISTORY Nuclear Medicine Begins with a Boa Constrictor
HISTORY Nuclear Medicine Begins with a Boa Constrictor Marshal! Brucer J Nucl Med 19: 581-598, 1978 In the beginning, a boa constrictor defecated in and then analyzed the insoluble precipitate. Just as London and the subsequent development of nuclear he suspected, it was almost pure (90.16%) uric medicine was inevitable. It took a little time, but the acid. As a thorough scientist he also determined the 139-yr chain of cause and effect that followed was "proportional number" of 37.5 for urea. ("Propor inexorable (7). tional" or "equivalent" weight was the current termi One June week in 1815 an exotic animal exhibi nology for what we now call "atomic weight.") This tion was held on the Strand in London. A young 37.5 would be used by Friedrich Woehler in his "animal chemist" named William Prout (we would famous 1828 paper on the synthesis of urea. Thus now call him a clinical pathologist) attended this Prout, already the father of clinical pathology, be scientific event of the year. While he was viewing a came the grandfather of organic chemistry. boa constrictor recently captured in South America, [Prout was also the first man to use iodine (2 yr the animal defecated and Prout was amazed by what after its discovery in 1814) in the treatment of thy he saw. The physiological incident was common roid goiter. He considered his greatest success the place, but he was the only person alive who could discovery of muriatic acid, inorganic HC1, in human recognize the material. Just a year earlier he had gastric juice. -

H*(10) Y Fluencias En Un Irradiador De Neutrones Con Una Fuente De Ra-Be
ISSSD 2020 ONLINE _________________________________________________________________________________ H*(10) y fluencias en un irradiador de neutrones con una fuente de 226Ra-Be Bedher O. Vega-Cabrera1,*, Héctor René Vega-Carrillo2, Víctor M. Viera Castillo1, César J. Guevara Pillaca1, Patrizia E., Pereyra Anaya1 María E. López Herrera1, Daniel F. Palacios Fernández1 1Pontificia Universidad Católica del Perú, Sección de Física. Av. Universitaria 1801, Apartado 1761, Lima – Perú. 2Universidad Autónoma de Zacatecas, Unidad Académica de Estudios Nucleares, C. Ciprés 10, Fracc. La Peñuela, 98068 Zacatecas, Zac. México. * E-mail: [email protected] Resumen Un irradiador de neutrones es un moderador con una fuente isotópica que es usado para enseñanza, entrenamiento y actividades de investigación. Normalmente, el moderador tiene puertos de irradiación radial y/o axial. Con el fin de utilizar el irradiador de neutrones de forma segura y óptima, deben conocerse los niveles de dosis y el espectro de la fluencia de neutrones. En este trabajo se utilizaron métodos Monte Carlo para estimar las fluencias de neutrones en tres rangos de energías: térmicos, epitérmicos y rápidos en siete puertos de un irradiador de neutrones con una fuente 226Ra-Be. El irradiador revestido de plomo contiene parafina wax como medio moderador de neutrones y sus puertos están asignados a diferentes distancias de la fuente de neutrones. El equivalente de dosis ambiental, debido a los neutrones, se estimó a 100 cm lateralmente y a 10 cm por encima del irradiador de neutrones. -

PGNAA Neutron Source Moderation Setup Optimization
Submitted to ‘Chinese Physics C PGNAA neutron source moderation setup optimization Zhang Jinzhao1(张金钊)Tuo Xianguo1(庹先国) (1.Chengdu University of Technology Applied Nuclear Techniques in Geoscience Key Laboratory of Sichuan Province,Chengdu 610059,China) Abstract: Monte Carlo simulations were carried out to design a prompt γ-ray neutron activation analysis (PGNAA) thermal neutron output setup using MCNP5 computer code. In these simulations the moderator materials, reflective materials and structure of the PGNAA 252Cf neutrons of thermal neutron output setup were optimized. Results of the calcuations revealed that the thin layer paraffin and the thick layer of heavy water moderated effect is best for 252Cf neutrons spectrum. The new design compared with the conventional neutron source design, the thermal neutron flux and rate were increased by 3.02 times and 3.27 times. Results indicate that the use of this design should increase the neutron flux of prompt gamma-ray neutron activation analysis significantly. Key word: PGNAA; neutron source; thermal neutron; moderation; reflection 1. Introduction study, Monte Carlo calculation was carried out for the Prompt gamma ray neutron activation analysis design of a 252Cf neutron source moderation setup for the (PGNAA) is a rapid, nondestructive, powerful analysis cement samples[7]. The model of Monte Carlo multielemental analysis technique, large samples of some simulation was verified by experiment[8, 9].We improve minor, trace light elements and is used in industrial the thermal neutron source yield rate of 252Cf neutron by control[1-5]. In a PGNAA analysis, the sample nuclear the PGNAA neutron source structure to the design. The composition is determined from prompt gamma rays calculation results for the new design were compared which produced through neutron inelastic scattering and with the previous, example: themal neutron flux rate, fast thermal neutron capture. -
![Arxiv:1506.05417V2 [Physics.Ins-Det] 28 Jul 2016](https://docslib.b-cdn.net/cover/1390/arxiv-1506-05417v2-physics-ins-det-28-jul-2016-561390.webp)
Arxiv:1506.05417V2 [Physics.Ins-Det] 28 Jul 2016
http://dx.doi.org/10.1016/j.apradiso.2016.06.032 A precise method to determine the activity of a weak neutron source using a germanium detector M. J. M. Dukea, A. L. Hallinb, C. B. Kraussb, P. Mekarskib,∗, L. Sibleyb aSLOWPOKE Nuclear Reactor Facility, University of Alberta, Edmonton, AB T6G 2G7, Canada bDepartment of Physics, University of Alberta, Edmonton, AB T6G 2E1, Canada Abstract A standard high purity germanium (HPGe) detector was used to determine the previously unknown neutron activity of a weak americium-beryllium (AmBe) neutron source. γ rays were created through 27Al(n,n0), 27Al(n,γ) and 1H(n,γ) reactions induced by the neutrons on aluminum and acrylic disks, respectively. These γ rays were measured using the HPGe detector. Given the unorthodox experimental arrangement, a Monte Carlo simulation was developed to model the efficiency of the detector system to determine the neutron activity from the measured γ rays. The activity of our neutron source was determined to be 307.4 ± 5.0 n/s and is consistent for the different neutron-induced γ rays. Keywords: neutron activation, germanium detector, simulation, spectroscopy, activity determination 1. Introduction As neutrons are difficult to detect, determining the absolute activity of a neutron source is challenging. This difficulty increases as the activity of the source decreases. Sophisticated techniques exist for neutron activity measure- ments, including the manganese bath technique[1], proton recoil techniques[2] and the use of 3He proportional counters[3]; nevertheless, the development of a method utilizing commonly available high purity germanium (HPGe) detectors would be advantageous. HPGe's are an industry standard for measuring γ ray energies to high preci- sion. -

Correlated Neutron Emission in Fission
Correlated neutron emission in fission S. Lemaire , P. Talou , T. Kawano , D. G. Madland and M. B. Chadwick ¡ Nuclear Physics group, Los Alamos National Laboratory, Los Alamos, NM, 87545 Abstract. We have implemented a Monte-Carlo simulation of the fission fragments statistical decay by sequential neutron emission. Within this approach, we calculate both the center-of-mass and laboratory prompt neutron energy spectra, the ¢ prompt neutron multiplicity distribution P ν £ , and the average total number of emitted neutrons as a function of the mass of ¢ the fission fragment ν¯ A £ . Two assumptions for partitioning the total available excitation energy among the light and heavy fragments are considered. Preliminary results are reported for the neutron-induced fission of 235U (at 0.53 MeV neutron energy) and for the spontaneous fission of 252Cf. INTRODUCTION Methodology In this work, we extend the Los Alamos model [1] by A Monte Carlo approach allows to follow in detail any implementing a Monte-Carlo simulation of the statistical reaction chain and to record the result in a history-type decay (Weisskopf-Ewing) of the fission fragments (FF) file, which basically mimics the results of an experiment. by sequential neutron emission. This approach leads to a We first sample the FF mass and charge distributions, much more detailed picture of the decay process and var- and pick a pair of light and heavy nuclei that will then de- ious physical quantities can then be assessed: the center- cay by emitting zero, one or several neutrons. This decay of-mass and laboratory prompt neutron energy spectrum sequence is governed by neutrons emission probabilities ¤ ¤ ¥ N en ¥ , the prompt neutron multiplicity distribution P n , at different temperatures of the compound nucleus and the average number of emitted neutrons as a function of by the energies of the emitted neutrons. -
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Radiation Safety Training
Radiation Safety Training …it concerns your health! 8/30/2006 Charlie Freeman, RSO Slide #1 SUNY Genseseo Atomic Structure Nucleus Orbiting Electrons – Contains Protons and – “Cloud” of orbiting Neutrons electrons surrounds nucleus – Cloud is relatively large – Small Size (~1E-14 m) (~1E-10 m) – Relatively Large Mass – Low mass – Extremely Large Density – Small amount of Stored – Large amount of Stored Energy Energy – Responsible for Chemical Bonds 8/30/2006 Charlie Freeman, RSO Slide #2 SUNY Genseseo Nomenclature • Element Designation A – “X” = Element Symbol X – “Z” = # protons in nucleus Z • “Atomic #” (each element has a unique Z, see periodic For Example table) 16 – “N” = # neutrons 8 O = O-16 = Oxygen 16 – Atomic mass # = “A” 17 • A = Z + N 8 O = O-17 = Oxygen 17 • Isotope: same Z, different N 197 and A 79 Au = Au-197 = Gold 197 8/30/2006 Charlie Freeman, RSO Slide #3 SUNY Genseseo Example: P-32 • 15 protons 32 • 17 neutrons P • A = 32 15 • Z =15 8/30/2006 Charlie Freeman, RSO Slide #4 SUNY Genseseo Ion • In an electrically neutral atom or molecule, the number of electrons equals the number of protons • Any atom or molecule with an imbalance in electrical charge is called an ion • Ions are chemically unstable, and will seek electrical neutrality by reacting with other atoms or molecules. 8/30/2006 Charlie Freeman, RSO Slide #5 SUNY Genseseo Radioactivity • Definition: A collection of unstable atoms that undergo spontaneous transformation that result in new elements. The degree of radioactivity is given by the number of decays that occur per unit time (decays per minute) • Units of measure: – Dpm, dps – Curie (Ci): 1 Ci = 3.7E10 dps – Bequerel (Bq): 1 Bq = 1dps 8/30/2006 Charlie Freeman, RSO Slide #6 SUNY Genseseo Half-Life & Decay Law • The activity of a sample of radioactive 225 200 atoms decreases over 175 150 time 125 100 75 Activity (Curies) • Half-life: how long it 50 25 takes for activity of 0 0 1 2 3 4 5 6 7 sample to decrease by Time (hours) a factor of ½. -

Neutron Sources
Neutron Sources Nadia Fomin Fundamental Neutron Physics Summer School Knoxville, TN 2015 Much content “borrowed” from Kevin Anderson, Mike Snow, Geoff 1 Greene, Scott Dewey, Mike Moncko, Jack Carpenter, and many others http://knoxbeerweek.com/ Neutron Sources Nadia Fomin Fundamental Neutron Physics Summer School Knoxville, TN 2015 Much content “borrowed” from Kevin Anderson, Mike Snow, Geoff 3 Greene, Scott Dewey, Mike Moncko, Jack Carpenter, and many others What is neutron physics ? Research which uses “low energy” neutrons from nuclear reactors and accelerator-driven spallation sources to address questions in nuclear, particle, and astrophysics Neutron Properties -22 Electric charge: qn=0, electrically neutral [qn<10 e] -5 -25 2 Size: rn~10 Angstrom=1 Fermi [area~ 10 cm =0.1 “barn”] Internal Structure: quarks [ddu, md~ mu~few MeV ] + gluons Spin: sn= 1/2 [Fermi statistics] Magnetic Dipole Moment: mn/ mp = -0.68497935(17) -26 Electric Dipole Moment: zero[dn < 10 e-cm] Mass: mn=939.566 MeV [mn> mp+ me, neutrons can decay] Lifetime: tn=880ish (depends on whom you ask) Neutrons are hard to get Neutrons are bound in nuclei, need several MeV for liberation E E=0 We want E~kT~25 meV (room temperature) VNN How to slow down a heavy neutral particle with Mn= Mp ? Lots of collisions… p n [1/2]N=(1 MeV)/(25 meV) for N collisions E 0 E/2 Neutrons are unstable when free->they can’t be accumulated easily Neutrons: Fast and Furious. And slow and gentle . Thermal ~25meV (2200m/s, λT=1.8Å) . Cold 50μeV-25meV . Very cold 2x10-7 - 5x10-5 eV .