Radionuclides Notice of Data Availability Technical Support Document
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
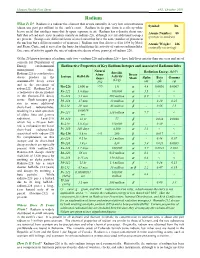
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

22Sra 226Ra and 222Rn in ' SOUTH CAROLINA GROUND WATER
Report No. 95 WRRI 22sRa 226Ra AND 222Rn IN ' SOUTH CAROLINA GROUND WATER: MEASUR~MEN-T TECHNIQUES AND ISOTOPE RELATIONSHIPS GlANNll\li FCUN~On o;: t,GR!CUL.TU~~tcoNOMlC2' ~~J.i\2Y J\li·iU ('lJ ~C 4· 1982 WATER RESOURCES RESEARCH INSTITUTE CLEMSON UNIVERSITY Clemson, .South Carolina JANUARY 1982 Water Resources Research Institute Clemson University Clemson, South Carolina 29631 ! 228Ra, 226Ra AND 222 Rn IN SOUTH CAROLINA GROUND WATER: MEASUREMENT TECHNIQUES AND ISOTOPE RELATIONSHIPS by Jacqueline Michel, Philip T. King and Willard S. Moore Department of Geology University of South Carolina Columbia, South Carolina 29208 The work upon which this puhlication is based was supported in part by funds provided by the Office of Water Research and Technology, project !!' No. OWRT-B-127-SC, U.S. Department of the Interior, fvashington, D. C., as authorized by the Water Research and Development Act of 1978 (PL95-467). r Project agreement No. 14-34-0001-9158 Period of Investigation: October 1979 - September 1980 Clemson University Water Resources Research Institute Technical Report No. 95 Contents of this publication do not necessarily reflect the views and policies of the Office of Water Research and Technology, U.S. Department of Interior, nor does mention of trade names or cormnercial products con stitute their endorsement or recommendation for use by the U.S. Goverrtment. ACKNOWLEDGEMENTS Much appreciation goes to Lewis Shaw, Rossie Stephens, Jim Ferguson and a large number of district personnel at the South Carolina Department of Health and Environmental Control who assisted us in field collection and provided much helpful information. Dr. -

Experimental Γ Ray Spectroscopy and Investigations of Environmental Radioactivity
Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity BY RANDOLPH S. PETERSON 216 α Po 84 10.64h. 212 Pb 1- 415 82 0- 239 β- 01- 0 60.6m 212 1+ 1630 Bi 2+ 1513 83 α β- 2+ 787 304ns 0+ 0 212 α Po 84 Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity Randolph S. Peterson Physics Department The University of the South Sewanee, Tennessee Published by Spectrum Techniques All Rights Reserved Copyright 1996 TABLE OF CONTENTS Page Introduction ....................................................................................................................4 Basic Gamma Spectroscopy 1. Energy Calibration ................................................................................................... 7 2. Gamma Spectra from Common Commercial Sources ........................................ 10 3. Detector Energy Resolution .................................................................................. 12 Interaction of Radiation with Matter 4. Compton Scattering............................................................................................... 14 5. Pair Production and Annihilation ........................................................................ 17 6. Absorption of Gammas by Materials ..................................................................... 19 7. X Rays ..................................................................................................................... 21 Radioactive Decay 8. Multichannel Scaling and Half-life ..................................................................... -

Analysis of Isotopes of Radium, Uranium and Thorium. 210Pb-210Po
Analysis of isotopes of radium, uranium and thorium. 210Pb-210Po Per Roos Risø National Laboratory for Sustainable Energy, Technical university of Denmark Radium isotopes Adsorption of Ra on MnO2 & direct alpha spectrometry Coating of polyamid (Nylon) with MnO2 (KMnO4 solution) ……half-life of Ra-224 is 88h Very thin MnO2 layer, poor yield but excellent resolution Electrodeposited sources Direct 20 days 6 months Analysis of 226Ra in water • Weigh up a suitable amount of water (10 litre is mostly sufficient). • Add HNO3 to pH 1-2. • Add 133Ba tracer (ca 10 Bq). • Add about 13 ml of a 0.2M KMnO4. • Adjust pH to about 8-9 with ammonia. • Add 15 ml 0.3M MnCl2 and stir for some 5 minutes. • Adjust with KMnO4 or MnCl2 to make the sample water phase slightly pink. • Collect the precipitate and centrifuge • Dissolve precipitate in 1M HCl-1%H2O2. • Add about 2g K2SO4+1ml H2SO4 and dilute to 100 ml with water. • Heat the sample to dissolve the K2SO4 completely. • Add 30 mg Pb2+. Heat for 20 minutes and stir. • Centrifuge the precipitate and discard supernate. • Wash precipitate with 100 ml of a solution of 0.2M H2SO4 and 0.1 M K2SO4 • Centrifuge the precipitate and discard supernate • Dissolve the PbSO4-precipitate with 3ml 0.1M EDTA at pH 10. • Transfer the solution to the scintillation vial (low diffusion PE-vial). • Wash centrifuge tube with 2 ml 0.1M EDTA and transfer to Scintillation vial. • Add 10ml OptiFluor 0 and fill with deionised water to total 20 ml. • Allow for ingrowth of 222Rn during 3 weeks and measure by LSQ. -

Naturally Occurring Radioactive Material (NORM VI) (NORM Material Radioactive Occurring Naturally Naturally Occurring Radioactive Material
Naturally Occurring Radioactive Material (NORM VI) Naturally Occurring Radioactive Material All minerals and raw materials contain radionuclides of natural origin. For (NORM VI) most human activities involving minerals and raw materials, the levels of exposure to these radionuclides are not significantly greater than normal background levels. However, certain work activities can give rise to signif- Proceedings icantly enhanced exposures that may need to be controlled by regulation. Material giving rise to these enhanced exposures has become known as of an International Symposium naturally occurring radioactive material (NORM). Historically, most regula- tory attention has been focused on the mining and processing of uranium Marrakesh, Morocco, 22–26 March 2010 ore, because such activities are a direct consequence of the radioactivity in the ore and form part of the nuclear fuel cycle. Over the past decade or two, however, more and more countries have introduced measures to reg- 1 ulate exposures arising from a wider range of natural sources. The NORM VI symposium, which was held in Marrakesh, Morocco, from 22–26 March 2010, provided an important opportunity to review the developments that had taken place during the three year period since the NORM V sym- posium in 2007. This period, which began with the publication of new radiation protection recommendations by the International Commission on Radiological Protection, was characterized by ongoing activities to revise international standards on radiation protection and safety. These Proceedings contain all 38 papers accepted for oral presentation and four rapporteur reports, as well as a summary that concludes with the main findings of the symposium. Text versions of 43 poster presentations are provided on a CD-ROM which accompanies these Proceedings. -

Occurrence of Radium-224, Radium
Prepared in cooperation with the New Jersey Department of Environmental Protection Occurrence of Radium-224, Radium-226 and Radium-228 in Water from the Vincentown and Wenonah-Mount Laurel Aquifers, the Englishtown Aquifer System, and the Hornerstown and Red Bank Sands, Southwestern and South-Central New Jersey Scientific Investigations Report 2007-5064 U.S. Department of the Interior U.S. Geological Survey Version 1.1 Occurrence of Radium-224, Radium-226 and Radium-228 in Water from the Vincentown and Wenonah-Mount Laurel Aquifers, the Englishtown Aquifer System, and the Hornerstown and Red Bank Sands, Southwestern and South-Central New Jersey By Vincent T. dePaul and Zoltan Szabo Prepared in cooperation with the New Jersey Department of Environmental Protection Scientific Investigations Report 2007–5064 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2007 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS--the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. -

Determination of Radium-228 in Natural Waters
Determination of Radium-228 in Natural Waters GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1696-G Determination of Radium-228 in Natural Waters By JESSE O. JOHNSON RADIOCHEMICAL ANALYSIS OF WATER GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1696-G UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1971 UNITED STATES DEPARTMENT OF THE INTERIOR ROGERS C. B. MORTON, Secretary GEOLOGICAL SURVEY William T. Pecora, Director Library of Congress catalog-card No. 72-610150 For sale by the Superintendent of Documents, U.S. Government Printing Office' Washington, D.C. 2O4O2 Price 25 cents (paper cover) CONTENTS Page Abstract ____________-______-_----------------------------------- Gl Introduction _______________---__-_------------------------------ 1 Review of radium-228 methods and studies -------------------- 5 Collection of water samples for radium-228 analysis ------------ 9 Development of the radium-228 method ---------------------------- 10 Natural radioactive nuclide interference ----------------------- 10 Radium-228 standard preparation ---------------------------- 13 Standards from "old" thorium nitrate ------------------------ 14 Standards from "old" thorium or thorium oxide _____-._-_-_---- 15 Standards from recently processed thorium reagents ----------- 15 Discussion of the method __.__-_---_-.__-_-__-------------------- 16 Apparatus ___._..__._______________.____-_-__------------------- 18 Reagents __________________---____--___---__-----_-------------- 18 Procedure for analysis of radium-228 ----------------------------- 20 Calculation of results ___-_--__---_--___-----_-----_-------------- 22 Errors and precision _______--_-_______-_-_-_-_--_---------------- 24 References ___.__.________________________________-__----------- 25 ILLUSTRATIONS Page FIGURE 1. Chart of the natural radioactive decay series ------------ G3 2. Graph of decay of airborne radioactivity trapped by membrane filters -______-_-----__--_-___--_------.-_- 12 TABLES Page TABLE 1. Radioactive element content of Colorado Front Range streams ____________________________________________ G8 2. -

Determination of Radium in Water
Determination of Radium in Water GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1696-B Determination of ladium in Water ; F. B. BARKER and J. 0. JOHNSON .ADIOCHEMICAL ANALYSIS OF WATER EOLOGICAL SURVEY WATER-SUPPLY PAPER 1696-B 'econd of a series of reports describing lethods used by the U.S. Geological 'urvey for measuring radioactivity nd radionuclides in water NITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1964 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 CONTENTS Abstract_____________----__-____-___________________ Bl Introduction-____________________________________________ 1 Methods for determining radium_________________________ ______ 2 Single-carrier-precipitation method._______________________ ____ 3 Principle of the method__________________________________ 3 Apparatus_______________________________________________ 4 Reagents._______________________ 7 Procedure._______________________________________________ 7 Techniques for handling difficult samples _____________________ 8 Calculation of results____________________________________ 8 Errors and precision________________________-__-__-__-_____ 9 Isotope-identification method.________________________ ______ 9 Principle of the method_____-_________-___-______________ 9 Apparatus. ___________________________ -___ 11 Reagents-___________________________-_______--__-_-----_- 11 Procedure. _____________.___________________________ -

Evaluation of Methods for the Assay of Radium-228 in Water
:^h3'?35 .1.1.^0^ ^^^'^^ °^ ^o, i NBS TECHNICAL NOTE 1137 °* V 1 y / NATL INST OF STAND & TECH A111D7 ED=^3flfl U.S. DEPARTMENT OF COMMERCE/ National Bureau of Standards Evaluation of Methods For the Assay of Radium-228 in Water QC 100 .U5753 NO. 1137 1981 C.2 NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act ot Congress on March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essential services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, mdustry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration services. The Laboratory consists of the following centers: Absolute Physical Quantities^ — Radiation Research — Thermodynamics and Molecular Science — Analytical Chemistry — Materials Science. -

The Radiochemistry of Radium Commllleeon NUCLEAR SCIENCE
National .—. Academy d Sciences NationalI Research Council m NUCLEAR SCIENCE SERIES The Radiochemistry of Radium COMMlllEEON NUCLEAR SCIENCE B.K- Aufmn,Chdmlm R. D. Evans, Wce Chairman UnlverOity of Chfcwo ~. In.Wttute of Technology Lewis slack,Secretary NstidFtem-cllmllntil E. c.Andmrmn HertwrtC?dd&tm i.mAlumnw. laboratory Calmibla Unlvertity C.J. BmkcwM J. J. Nlckmn M Ridge MU. Iabmntm-y NEmOrial Eo9pltd (New York) RobertG.cm- IL L. Phtzma!l A& MColbWaf TcmO A~ N81icad la.tamtory ugoFam D. M Vm P+ter NaUOadwruofsmldmda Eartol RO~ Fwdmon Oeorgew.Wetbefill Urdvarslty of CUlfonda (Ian Angeles) LIAISONMRMBERS padC. Aaberwld JeromeRegean AtOmlcEwrgy-mmfmtm UfIce ofNaml Reach Ja6ePh E. Duval J. &wwd McMlflen Afr Farce OfUce of SctenUflc ~h NuUc4ml Sdence FwndaUm SIJ9COMMlllEEON RADI-RMISTRY N. E. -, Chd- J. D. Kn@t u.& NavalFwlblO@dDOfenlm ICaAlamwSelmtlfic I.atmratmy Lab0rnk3m J. M. Nielmn G. R CbqPln 06netal Electric Company (IuChiand) FlOrMn State Uutvertiw G. D. O’ftdley E. M Clark W NMge National Laboratory Rmaselaer POlytadmlc fmtitute RP. tldmman R. M“ Dimnold AtOmlc mml’w rnvistml Lawrence Radiaklm Iabm-story Pfltlllpa Petroleum COi+my (LillM FalM) A.W.Fdrf@l K P. ~rg Unlversfty of Washl@m Argmne NatfOllaf mbodmy Jerome Ndls P. c.Wevanean Brcakkav671 Natfanal fdwratory Lawrence lwnatlan LabOrafov D. N. bhiernmn Mttelb Mernorhf fmlltute coNsLaTANl J. W. Ulncbetier “~wata hlefmuti of 1%~ ,. The Radiochemistry of Radium By H. W. Kirby Mound Laboratory Monsanto Research Corporation Miamisburg, Ohio and Murrell L. Salutsky W. R. Grace & Co. Research Division Washington Research Center Clarksville, Md. kued,December19&4 Subcommitteeon Radiochemistry NationalAcademy of Sciences—NaUonal Research Council Prin&dinUSA.Price$2.25Av-dhblefromtieClear4@cmseforFederal Sciendficu@ TechnknlInfonnatlon,Nation8.1BureauofStaxhr&,U.S.De- parbnentofComme-,SPri@ield,Vir@ia. -

Radionuclides
Physics of Novel Radiation Modalities: Radionuclides James S. Welsh Stritch School of Medicine Loyola University Chicago Disclosure • Member of the Advisory Committee on the Medical Uses of Isotopes (ACMUI) for the United States Nuclear Regulatory Commission (NRC) • Board of directors: – Coqui Radioisotopes – Colossal Fossils Learning Objectives • Understand the basic physics of alpha, beta, gamma and other types of radioactivity • Gain some familiarity with the various sealed and unsealed radionuclides commonly used in radiation oncology Types of radioactivity • Alpha • Beta – Beta minus – beta plus (positron emission) – electron capture • Gamma – Isomeric transitions – Internal conversion – Internal pair production • Cluster radioactivity • Spontaneous fission – Binary or ternary • Rare types: – Proton radioactivity – b+ delayed proton emission – b- delayed neutron emission – b+ delayed deuteron or triton emission – Beta delayed fission Fun with Isotopes Radioactive decay supposedly follows a mathematically precise exponential function • Supposedly unaffected by temperature, pressure, chemical environment • First declared by Rutherford, Chadwick and Ellis Generally true but… …well-known exceptions do exist …well-known exceptions do exist • Electron Capture (e.g. 7Be, 109In, 110Sn) – If chemical environment make K-shell electrons less accessible, decay rate might be altered …well-known exceptions do exist • Electron Capture (e.g. 7Be, 109In, 110Sn) – If chemical environment make K-shell electrons less accessible, decay rate might be altered -

Radium-226, Releases Half of Its Radiation in About 1,600 Years
TOXICOLOGICAL PROFILE FOR RADIUM Agency for Toxic Substances and Disease Registry U.S. Public Health Service In collaboration with: U.S. Environmental Protection Agency December 1990 ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorbement by the Agency for Toxic Substances and Disease Registry. 1 1. PUBLIC HEALTH STATEMENT This Statement was prepared to give you information about radium and to emphasize the human health effects that may result from exposure to it. The Environmental Protection Agency (EPA) has identified 1,177 sites on its National Priorities List (NPL). Radium has been found above background levels at 18 of these sites. However, we do not know how many of the 1,177 NPL sites have been evaluated for radium. As EPA evaluates more sites, the number of sites at which radium is found above background levels may change. The information is important for you because radium may cause harmful health effects and because these sites are potential or actual sources of human exposure to radium. When a radioactive chemical is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment as a radioactive chemical emission. This emission, which is also called a release, does not always lead to exposure. You can be exposed to a radioactive chemical when you come into contact with that chemical alone or with a substance that contains it. You may be exposed to it in the environment by breathing, eating, or drinking substances containing the radioactive chemical or from skin contact with it.