Radium-226, Releases Half of Its Radiation in About 1,600 Years
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Strontium-90
EPA Facts about Strontium-90 What is strontium-90? The most common isotope of strontium is strontium-90. Radioactive strontium-90 is produced when uranium and plutonium undergo fission. Fission The time required for a radioactive substance to is the process in which the nucleus of a lose 50 percent of its radioactivity by decay is radionuclide breaks into smaller parts. Large known as the half-life. Strontium-90 has a half- amounts of radioactive strontium-90 were life of 29 years and emits beta particles of produced during atmospheric nuclear weapons relatively low energy as it decays. Yttrium-90, its tests conducted in the 1950s and 1960s. As a decay product, has a shorter half-life (64 hours) result of atmospheric testing and radioactive than strontium-90, but it emits beta particles of fallout, this strontium was dispersed and higher energy. deposited on the earth. How are people exposed to strontium- 90? What are the uses of strontium-90? Although external exposure to strontium-90 Strontium-90 is used in medical and agricultural from nuclear testing is of minor concern because studies. It is also used in thermoelectric devices environmental concentrations are low, that are built into small power supplies for use strontium in the environment can become part of the food chain. This pathway of exposure in remote locations, such as navigational beacons, remote weather stations, and space became a concern in the 1950s with the advent vehicles. Additionally, strontium-90 is used in of atmospheric testing of nuclear explosives. electron tubes, radioluminescent markers, as a With the suspension of atmospheric testing of radiation source in industrial thickness gauges, nuclear weapons, dietary intake has steadily and for treatment of eye diseases. -
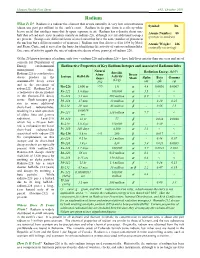
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -
![(12) United States Patent (10) Patent N0.: US 7,887,782 B2 Schwarz Et A]](https://docslib.b-cdn.net/cover/9007/12-united-states-patent-10-patent-n0-us-7-887-782-b2-schwarz-et-a-89007.webp)
(12) United States Patent (10) Patent N0.: US 7,887,782 B2 Schwarz Et A]
US007887782B2 (12) United States Patent (10) Patent N0.: US 7,887,782 B2 Schwarz et a]. (45) Date of Patent: Feb. 15, 2011 (54) RADIOTHERAPEUTIC FORMULATIONS 4,859,431 A * 8/1989 Ehrhardt ............. .. 250/432 PD CONTAINING 224RA AND A METHOD FOR 5,457,323 A 10/1995 Geerlings THEIR PRODUCTION 5,854,968 A * 12/1998 Horwitz et a1. .............. .. 423/2 (75) Inventors: UWe SchWarz, Salzgitter (DE); Rolf Daniels, Salzgitter (DE) OTHER PUBLICATIONS Delikan, O. Health Phys. 1978, 35, 21-24.* (73) Assignee: Altmann Therapie GmbH & Co., Narbutt et al. (Appl. Radiat. lsot. 1998, 49, 89-91).* Salzgitter (DE) Orhan Delikan; Preparation of 224Ra for Therapy of Ankylosing Sp0ndylitis*; Health Physics Vol. 35 (July) pp. 21-24 Pergamon ( * ) Notice: Subject to any disclaimer, the term of this Press Ltd., 1978. patent is extended or adjusted under 35 Radioaktives Arzneimittel; Gebrauchsinformation und Fachinforma U.S.C. 154(b) by 671 days. tion; pp. 1-6, N0 English translation. Alberding, A. et al., “Wirksamkeit und Vertraglichkeit von (21) Appl. No.: 10/362,049 Radiumchlorid in der Behandlung der ankylo sierenden Spondylitis,” Z Rheumatol 2006, 65:245-251. (22) PCT Filed: Aug. 7, 2001 * cited by examiner (86) PCT No.: PCT/EP01/09121 Primary ExamineriMichael G Hartley Assistant ExamineriMelissa Perreira § 371 (0X1)’ (74) Attorney, Agent, or FirmiSam K. Tahmassebi; (2), (4) Date: Jul. 9, 2003 TechLaW LLP (87) PCT Pub. No.: WO02/15943 (57) ABSTRACT PCT Pub. Date: Feb. 28, 2002 The invention relates to novel radiotherapeutic formulations (65) Prior Publication Data containing 224Ra and methods for their production. The US 2004/0028606 A1 Feb. -

22Sra 226Ra and 222Rn in ' SOUTH CAROLINA GROUND WATER
Report No. 95 WRRI 22sRa 226Ra AND 222Rn IN ' SOUTH CAROLINA GROUND WATER: MEASUR~MEN-T TECHNIQUES AND ISOTOPE RELATIONSHIPS GlANNll\li FCUN~On o;: t,GR!CUL.TU~~tcoNOMlC2' ~~J.i\2Y J\li·iU ('lJ ~C 4· 1982 WATER RESOURCES RESEARCH INSTITUTE CLEMSON UNIVERSITY Clemson, .South Carolina JANUARY 1982 Water Resources Research Institute Clemson University Clemson, South Carolina 29631 ! 228Ra, 226Ra AND 222 Rn IN SOUTH CAROLINA GROUND WATER: MEASUREMENT TECHNIQUES AND ISOTOPE RELATIONSHIPS by Jacqueline Michel, Philip T. King and Willard S. Moore Department of Geology University of South Carolina Columbia, South Carolina 29208 The work upon which this puhlication is based was supported in part by funds provided by the Office of Water Research and Technology, project !!' No. OWRT-B-127-SC, U.S. Department of the Interior, fvashington, D. C., as authorized by the Water Research and Development Act of 1978 (PL95-467). r Project agreement No. 14-34-0001-9158 Period of Investigation: October 1979 - September 1980 Clemson University Water Resources Research Institute Technical Report No. 95 Contents of this publication do not necessarily reflect the views and policies of the Office of Water Research and Technology, U.S. Department of Interior, nor does mention of trade names or cormnercial products con stitute their endorsement or recommendation for use by the U.S. Goverrtment. ACKNOWLEDGEMENTS Much appreciation goes to Lewis Shaw, Rossie Stephens, Jim Ferguson and a large number of district personnel at the South Carolina Department of Health and Environmental Control who assisted us in field collection and provided much helpful information. Dr. -

Virulence and Transcriptome Profile of Multidrug-Resistant Escherichia Coli from Chicken Received: 3 April 2017 Hafiz I.Hussain 1, Zahid Iqbal1,4, Mohamed N
www.nature.com/scientificreports OPEN Virulence and transcriptome profile of multidrug-resistant Escherichia coli from chicken Received: 3 April 2017 Hafiz I.Hussain 1, Zahid Iqbal1,4, Mohamed N. Seleem3, Deyu Huang2, Adeel Sattar2, Haihong Accepted: 3 July 2017 Hao1 & Zonghui Yuan1,2 Published: xx xx xxxx Numerous studies have examined the prevalence of pathogenic Escherichia coli in poultry and poultry products; however, limited data are available regarding their resistance- and virulence-associated gene expression profiles. This study was designed to examine the resistance and virulence of poultryE. coli strains in vitro and in vivo via antibiotic susceptibility, biofilm formation and adhesion, and invasion and intracellular survivability assays in Caco-2 and Raw 264.7 cell lines as well as the determination of the median lethal dose in two-day old chickens. A clinical pathogenic multidrug-resistant isolate, E. coli 381, isolated from broilers, was found to be highly virulent in cell culture and 1000-fold more virulent in a chicken model than other strains; accordingly, the isolate was subsequently selected for transcriptome analysis. The comparative gene expression profile of MDRE. coli 381 and the reference human strain E. coli ATCC 25922 was completed with Illumina HiSeq. 2500 transcriptome analysis. Differential gene expression analysis indicates that there are multiple pathways involved in the resistance and virulence of this highly virulent strain. The results garnered from this study provide critical information about the highly virulent MDR E. coli strain of poultry origin and warrant further investigation due to its significant threat to public health. Escherichia coli is a Gram-negative bacterium that displays a wide range of genomic diversity. -

What Is Radon?
Biology Unit Radon Alert INVESTIGATION 3 WHAT IS RADON? INTRODUCTION Radon is a naturally occurring radioactive gas. It is formed by the radioactive breakdown of radium, and is found in soils just about everywhere. You cannot see it, taste it, or smell it. It is continuously formed in rocks and soils and escapes into the atmosphere. In some cases, it makes its way into homes, builds up to high concentrations in indoor air, and can become a health hazard. Although there are several different isotopes of radon, the one that is of greatest concern as a potential Radioactivity - the spontaneous human health threat is called radon-222. Radon-222 is emission of energy by certain (radio- formed naturally during a chain of radioactive disintegra- active) atoms, resulting in a change tion reactions (decay series). The decay series begins from one element to another or one when uranium-238 decays. Uranium is widely distrib- isotope to another. The energy can uted in rocks and soils throughout the earth’s crust. It has be in the form of alpha or beta par- a half-life of 4.5 billion years, which means a very slow ticles and gamma rays. breakdown. The decay series is shown schematically in Figure 1. There are eight different elements and 15 different isotopes in the series, beginning with uranium- 238 and ending with lead-206. New elements formed by radioactive disintegration reactions are called decay products. Thus, radium-226 is one of the decay products of uranium-238. Polonium-218 and lead-214 are decay products of radon-222. -

Experimental Γ Ray Spectroscopy and Investigations of Environmental Radioactivity
Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity BY RANDOLPH S. PETERSON 216 α Po 84 10.64h. 212 Pb 1- 415 82 0- 239 β- 01- 0 60.6m 212 1+ 1630 Bi 2+ 1513 83 α β- 2+ 787 304ns 0+ 0 212 α Po 84 Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity Randolph S. Peterson Physics Department The University of the South Sewanee, Tennessee Published by Spectrum Techniques All Rights Reserved Copyright 1996 TABLE OF CONTENTS Page Introduction ....................................................................................................................4 Basic Gamma Spectroscopy 1. Energy Calibration ................................................................................................... 7 2. Gamma Spectra from Common Commercial Sources ........................................ 10 3. Detector Energy Resolution .................................................................................. 12 Interaction of Radiation with Matter 4. Compton Scattering............................................................................................... 14 5. Pair Production and Annihilation ........................................................................ 17 6. Absorption of Gammas by Materials ..................................................................... 19 7. X Rays ..................................................................................................................... 21 Radioactive Decay 8. Multichannel Scaling and Half-life ..................................................................... -

On the Solubility of Radium Sulfate and Carbonate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Chalmers Publication Library THESIS FOR THE DEGREE OF LICENTIATE OF ENGINEERING On the solubility of radium sulfate and carbonate Artem Vasilyevich Matyskin Nuclear Chemistry Department of Chemistry and Chemical Engineering CHALMERS UNIVERSITY OF TECHNOLOGY Gothenburg, Sweden 2016 On the solubility of radium sulfate and carbonate © ARTEM VASILYEVICH MATYSKIN, 2016 Technical report number: 2016:04 ISSN number: 1652-943X Nuclear Chemistry Department of Chemistry and Chemical Engineering Chalmers University of Technology SE – 412 96 Göteborg Sweden Telephone +46(0)31-772 1000 Cover: Radium sulfate powder Chalmers Reproservice Gothenburg, Sweden 2016 On the solubility of radium sulfate and carbonate Artem V. Matyskin Nuclear Chemistry Department of Chemistry and Chemical Engineering Chalmers University of Technology Abstract Radium is one of the most toxic elements and its concentration in different human activities and migration from man-made wastes provokes a strong interest in environmental science. To be able to model the migration process, reliable experimental thermodynamic data of radium compounds are needed. In this work details of the safe radium source disassembly which were previously used in brachytherapy are described and different methods for conversion of RaSO4 into aqueous solution are reviewed. The method of choice included three cycles of RaSO4 heating in 1.5 M Na2CO3 up to 85 ºC, cooling and subsequent removal of supernatant. X-ray diffraction studies showed that the method allows the synthesis of amorphous RaCO3, which can be dissolved in mineral acid. Gamma spectrometric measurements showed that most of the initial RaSO4 was 210 converted into solution and that 7 ± 1 % of the initial Pb was co-precipitated with RaCO3. -

DOCUMENT Mute
DOCUMENT mute ED 124 18 . 88 SE 019 612 TITLEN: Radioactivity .and Man iinicourse, Career Oriented Pre-Technical Physics* , INSTITUTI N Dallas Independent School District, Tex. SPONS AGE CY Bureauvof Ele*entary and Secondary tducat4.0n (D5 HEN/OE),-WashingtOn, D.C. -RUB DATE 7 For rilated 0, NOTE - 78p.; Drawings may not reproduce wel documens, see SE 018 322-333 and SE 019 605-616 , . 4 EDRS PRICE MF-$0.83 HC-$4.67 Plus Postag4. DESCRIPTOR Instructional Materialsi Physics; *Program Guides; *Radiation Effects; *Science Activities; Science .41 Careers; Science Education; *Science Materials; Secondary Zdution;.*Secondary School Science; Technical Educe ion IDENTIFIrRS Elementary Seco dart' VIAlcation ACt Title III; ESEA Title' III 07. 1 ABSTRACT .1j This instructional guide, intended for student use, develops the subject of radioactivity and can through a series. of sequential activities.. `A technical development of the subject is pursued with examples stressing practical aspects'of the concepts. Included in the minicourse are:, (1) the rationale,(2) terminal behavioral objectives, (3) enabling behavioral objectives,(4) activities,(5) resource ,packages, and (6) evaluation materials. The' * benefits as well as the dangers of radiometivity to Ian are considered.,This unit is one of twelve intended for use in.the second year of tv9 year vocationally oriented .physics. program. (CP) j t I J ***********************************************************************. * . Documents acquired by ERIC include many inforgal unpublished * materials not a40.1able.from other sources. ERIC makes every effort * * to obtiit the best copy available. Nevertheless,items of.marginel * *.reproducibility are often encountered and this-affects the quality * - *`of the microfiche and hardcopy reproductions ERIC makes available * *. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

Oecd Guideline for the Testing of Chemicals
Draft November 2009 OECD GUIDELINE FOR THE TESTING OF CHEMICALS PROPOSAL FOR A NEW TEST GUIDELINE 223 Avian Acute Oral Toxicity Test INTRODUCTION 1. This test guideline describes procedures designed to estimate the acute oral toxicity of substances to birds, and it provides three testing options: (1) limit dose test, (2) LD50-slope test, and (3) LD50-only test. The LD50-slope and LD50-only options are sequential testing procedures. The test method selected will depend on whether or not a definitive median dose (LD50) and slope of the dose-response curve are both needed. Sequential testing procedures target the placement of doses and match the precision of the endpoint with the precision required. These sequential procedures were designed to minimise the numbers of birds used. A computer programme is available to aid the placement of doses and estimate the LD50, slope and confidence limits. 2. Development of this test guideline began at the SETAC/OECD Workgroup on avian toxicity testing following a workshop held in Pensacola, Florida, United States, in 1994 (1) with subsequent open SETAC and closed OECD Expert Group meetings in Europe and the United States to develop and optimise the sequential testing design. The sequential testing design has been developed with extensive statistical validation (2). INITIAL CONSIDERATIONS 3. The information required by different hazard assessment schemes may vary considerably. To satisfy these various needs, the following three tests are described: Limit dose test – This is the preferred test when toxicity is expected to be low and lethality is unlikely at the limit dose. The limit dose must be adequate for assessment purposes, and it is usually 2000 mg/kg-bwt. -

Toxicological Profile for Radon
RADON 205 10. GLOSSARY Some terms in this glossary are generic and may not be used in this profile. Absorbed Dose, Chemical—The amount of a substance that is either absorbed into the body or placed in contact with the skin. For oral or inhalation routes, this is normally the product of the intake quantity and the uptake fraction divided by the body weight and, if appropriate, the time, expressed as mg/kg for a single intake or mg/kg/day for multiple intakes. For dermal exposure, this is the amount of material applied to the skin, and is normally divided by the body mass and expressed as mg/kg. Absorbed Dose, Radiation—The mean energy imparted to the irradiated medium, per unit mass, by ionizing radiation. Units: rad (rad), gray (Gy). Absorbed Fraction—A term used in internal dosimetry. It is that fraction of the photon energy (emitted within a specified volume of material) which is absorbed by the volume. The absorbed fraction depends on the source distribution, the photon energy, and the size, shape and composition of the volume. Absorption—The process by which a chemical penetrates the exchange boundaries of an organism after contact, or the process by which radiation imparts some or all of its energy to any material through which it passes. Self-Absorption—Absorption of radiation (emitted by radioactive atoms) by the material in which the atoms are located; in particular, the absorption of radiation within a sample being assayed. Absorption Coefficient—Fractional absorption of the energy of an unscattered beam of x- or gamma- radiation per unit thickness (linear absorption coefficient), per unit mass (mass absorption coefficient), or per atom (atomic absorption coefficient) of absorber, due to transfer of energy to the absorber.