Summary of Data Reported and Evaluation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
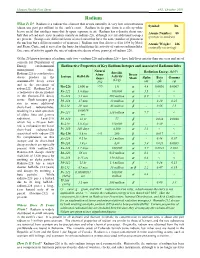
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

22Sra 226Ra and 222Rn in ' SOUTH CAROLINA GROUND WATER
Report No. 95 WRRI 22sRa 226Ra AND 222Rn IN ' SOUTH CAROLINA GROUND WATER: MEASUR~MEN-T TECHNIQUES AND ISOTOPE RELATIONSHIPS GlANNll\li FCUN~On o;: t,GR!CUL.TU~~tcoNOMlC2' ~~J.i\2Y J\li·iU ('lJ ~C 4· 1982 WATER RESOURCES RESEARCH INSTITUTE CLEMSON UNIVERSITY Clemson, .South Carolina JANUARY 1982 Water Resources Research Institute Clemson University Clemson, South Carolina 29631 ! 228Ra, 226Ra AND 222 Rn IN SOUTH CAROLINA GROUND WATER: MEASUREMENT TECHNIQUES AND ISOTOPE RELATIONSHIPS by Jacqueline Michel, Philip T. King and Willard S. Moore Department of Geology University of South Carolina Columbia, South Carolina 29208 The work upon which this puhlication is based was supported in part by funds provided by the Office of Water Research and Technology, project !!' No. OWRT-B-127-SC, U.S. Department of the Interior, fvashington, D. C., as authorized by the Water Research and Development Act of 1978 (PL95-467). r Project agreement No. 14-34-0001-9158 Period of Investigation: October 1979 - September 1980 Clemson University Water Resources Research Institute Technical Report No. 95 Contents of this publication do not necessarily reflect the views and policies of the Office of Water Research and Technology, U.S. Department of Interior, nor does mention of trade names or cormnercial products con stitute their endorsement or recommendation for use by the U.S. Goverrtment. ACKNOWLEDGEMENTS Much appreciation goes to Lewis Shaw, Rossie Stephens, Jim Ferguson and a large number of district personnel at the South Carolina Department of Health and Environmental Control who assisted us in field collection and provided much helpful information. Dr. -

Experimental Γ Ray Spectroscopy and Investigations of Environmental Radioactivity
Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity BY RANDOLPH S. PETERSON 216 α Po 84 10.64h. 212 Pb 1- 415 82 0- 239 β- 01- 0 60.6m 212 1+ 1630 Bi 2+ 1513 83 α β- 2+ 787 304ns 0+ 0 212 α Po 84 Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity Randolph S. Peterson Physics Department The University of the South Sewanee, Tennessee Published by Spectrum Techniques All Rights Reserved Copyright 1996 TABLE OF CONTENTS Page Introduction ....................................................................................................................4 Basic Gamma Spectroscopy 1. Energy Calibration ................................................................................................... 7 2. Gamma Spectra from Common Commercial Sources ........................................ 10 3. Detector Energy Resolution .................................................................................. 12 Interaction of Radiation with Matter 4. Compton Scattering............................................................................................... 14 5. Pair Production and Annihilation ........................................................................ 17 6. Absorption of Gammas by Materials ..................................................................... 19 7. X Rays ..................................................................................................................... 21 Radioactive Decay 8. Multichannel Scaling and Half-life ..................................................................... -

Analysis of Isotopes of Radium, Uranium and Thorium. 210Pb-210Po
Analysis of isotopes of radium, uranium and thorium. 210Pb-210Po Per Roos Risø National Laboratory for Sustainable Energy, Technical university of Denmark Radium isotopes Adsorption of Ra on MnO2 & direct alpha spectrometry Coating of polyamid (Nylon) with MnO2 (KMnO4 solution) ……half-life of Ra-224 is 88h Very thin MnO2 layer, poor yield but excellent resolution Electrodeposited sources Direct 20 days 6 months Analysis of 226Ra in water • Weigh up a suitable amount of water (10 litre is mostly sufficient). • Add HNO3 to pH 1-2. • Add 133Ba tracer (ca 10 Bq). • Add about 13 ml of a 0.2M KMnO4. • Adjust pH to about 8-9 with ammonia. • Add 15 ml 0.3M MnCl2 and stir for some 5 minutes. • Adjust with KMnO4 or MnCl2 to make the sample water phase slightly pink. • Collect the precipitate and centrifuge • Dissolve precipitate in 1M HCl-1%H2O2. • Add about 2g K2SO4+1ml H2SO4 and dilute to 100 ml with water. • Heat the sample to dissolve the K2SO4 completely. • Add 30 mg Pb2+. Heat for 20 minutes and stir. • Centrifuge the precipitate and discard supernate. • Wash precipitate with 100 ml of a solution of 0.2M H2SO4 and 0.1 M K2SO4 • Centrifuge the precipitate and discard supernate • Dissolve the PbSO4-precipitate with 3ml 0.1M EDTA at pH 10. • Transfer the solution to the scintillation vial (low diffusion PE-vial). • Wash centrifuge tube with 2 ml 0.1M EDTA and transfer to Scintillation vial. • Add 10ml OptiFluor 0 and fill with deionised water to total 20 ml. • Allow for ingrowth of 222Rn during 3 weeks and measure by LSQ. -

Radiological Implications of Plutonium Recycle and the Use of Thorium Fuels in Power Reactor Operations
01 RD/B/N3523 Central Electricity Generating Board Research Department Berkeley Nuclear Laboratories RADIOLOGICAL IMPLICATIONS OF PLUTONIUM RECYCLE AND THE USE OF THORIUM FUELS IN POWER REACTOR OPERATIONS By H. F. Macdonald XJ034 January 1976 DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. Radiological Implications of Plutonium Recycle and the Use of Thorium Fuels in Power Reactor Operations “ by - H.F. Macdonald Approved Head of Health Physics Research Section For inclusion in Nuclear Science Abstracts SUMMARY As economically attractive sources of natural uranium are gradually depleted attention will turn to recycling plutonium or the use of thorium fuels. In this study the radiological implications of these fuel cycles in terms of fuel handling and radioactive waste disposal are investigated in 235 relation to a conventional U enriched oxide fuel. It is suggested that a comparative study of this nature may be an important aspect of the overall optimisation of future fuel cycle strategies. It is shown that the use of thorium based fuels has distinct advan tages in terms of neutron dose rates from irradiated fuels and long term a decay heating commitment compared with conventional uranium/plutonium fuels. However, this introduces a y dose rate problem in the fabrication 233 and handling of unirradiated U fuels. For both plutonium and thorium fuels these radiological problems increase during storage of the fuel prior to reactor irradiation. Finally, the novel health physics problems which arise in the handling and processing of thorium fuels are reviewed in an appendix. -

Naturally Occurring Radioactive Material (NORM VI) (NORM Material Radioactive Occurring Naturally Naturally Occurring Radioactive Material
Naturally Occurring Radioactive Material (NORM VI) Naturally Occurring Radioactive Material All minerals and raw materials contain radionuclides of natural origin. For (NORM VI) most human activities involving minerals and raw materials, the levels of exposure to these radionuclides are not significantly greater than normal background levels. However, certain work activities can give rise to signif- Proceedings icantly enhanced exposures that may need to be controlled by regulation. Material giving rise to these enhanced exposures has become known as of an International Symposium naturally occurring radioactive material (NORM). Historically, most regula- tory attention has been focused on the mining and processing of uranium Marrakesh, Morocco, 22–26 March 2010 ore, because such activities are a direct consequence of the radioactivity in the ore and form part of the nuclear fuel cycle. Over the past decade or two, however, more and more countries have introduced measures to reg- 1 ulate exposures arising from a wider range of natural sources. The NORM VI symposium, which was held in Marrakesh, Morocco, from 22–26 March 2010, provided an important opportunity to review the developments that had taken place during the three year period since the NORM V sym- posium in 2007. This period, which began with the publication of new radiation protection recommendations by the International Commission on Radiological Protection, was characterized by ongoing activities to revise international standards on radiation protection and safety. These Proceedings contain all 38 papers accepted for oral presentation and four rapporteur reports, as well as a summary that concludes with the main findings of the symposium. Text versions of 43 poster presentations are provided on a CD-ROM which accompanies these Proceedings. -

Properties of Selected Radioisotopes
CASE FILE COPY NASA SP-7031 Properties of Selected Radioisotopes A Bibliography PART I: UNCLASSIFIED LITERATURE NATIONAL AERONAUTICS AND SPACE ADMINISTRATION NASA SP-7031 PROPERTIES OF SELECTED RADIOISOTOPES A Bibliography Part I: Unclassified Literature A selection of annotated references to technical papers, journal articles, and books This bibliography was compiled and edited by DALE HARRIS and JOSEPH EPSTEIN Goddard Space Flight Center Greenbelt, Maryland Scientific and Technical Information Division / OFFICE OF TECHNOLOGY UTILIZATION 1968 USP. NATIONAL AERONAUTICS AND SPACE ADMINISTRATION Washington, D.C. PREFACE The increasing interest in the application of substantial quantities of radioisotopes for propulsion, energy conversion, and various other thermal concepts emphasizes a need for the most recent and most accurate information available describing the nuclear, chemical, and physical properties of these isotopes. A substantial amount of progress has been achieved in recent years in refining old and developing new techniques of measurement of the properties quoted, and isotope processing. This has resulted in a broad technological base from which both the material and information about the material is available. Un- fortunately, it has also resulted in a multiplicity of sources so that information and data are either untimely or present properties without adequately identifying the measurement techniques or describing the quality of material used. The purpose of this document is to make available, in a single reference, an annotated bibliography and sets of properties for nine of the more attractive isotopes available for use in power production. Part I contains all the unclassified information that was available in the literature surveyed. Part II is the classified counterpart to Part I. -

Radionuclides Notice of Data Availability Technical Support Document
Radionuclides Notice of Data Availability Technical Support Document March 2000 Targeting and Analysis Branch Standards and Risk Management Division Office of Ground Water and Drinking Water United States Environmental Protection Agency Prepared by: USEPA Office of Ground Water and Drinking Water in collaboration with USEPA Office of Indoor Air and Radiation United States Geological Survey Preface Under the authority of the Safe Drinking Water Act (SDWA) and its amendments, EPA sets drinking water standards for contaminants, which potentially pose a threat to public health via a public drinking water source. In 1976, EPA promulgated National Interim Primary Drinking Water Regulations (NIPDWR) for three categories of radionuclides: gross alpha emitters, radium 226 and 228 combined, and gross beta and photon emitters. The 1976 NIPDWRs for these radionuclides regulated: 1. gross alpha at 15 picoCuries per liter (pCi/L)(excluding radon and uranium); 2. radium-226 and radium-228 combined at 5 pCi/L; 3. and beta/photon emitters at a 4 millirem dose of radioactivity. The 1986 reauthorization of the Safe Drinking Water Act (SDWA) declared the interim standards for these radionuclides to be final National Primary Drinking Water Regulations (NPDWR). The 1986 amendment also required standards to be set as close to the maximum contaminant level goal (MCLG; the health goal) as possible. At that time, radionuclides did not have an MCLG. In 1991 (56 FR 33050), EPA proposed: < A Maximum Contaminant Level Goal (MCLG) of zero for all radionuclides; < A -

Occurrence of Radium-224, Radium
Prepared in cooperation with the New Jersey Department of Environmental Protection Occurrence of Radium-224, Radium-226 and Radium-228 in Water from the Vincentown and Wenonah-Mount Laurel Aquifers, the Englishtown Aquifer System, and the Hornerstown and Red Bank Sands, Southwestern and South-Central New Jersey Scientific Investigations Report 2007-5064 U.S. Department of the Interior U.S. Geological Survey Version 1.1 Occurrence of Radium-224, Radium-226 and Radium-228 in Water from the Vincentown and Wenonah-Mount Laurel Aquifers, the Englishtown Aquifer System, and the Hornerstown and Red Bank Sands, Southwestern and South-Central New Jersey By Vincent T. dePaul and Zoltan Szabo Prepared in cooperation with the New Jersey Department of Environmental Protection Scientific Investigations Report 2007–5064 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2007 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS--the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. -

ORAU Team Document Number: ORAUT-TKBS-0016-5
ORAU Team Document Number: ORAUT-TKBS-0016-5 Dose Reconstruction Project for NIOSH Effective Date: 09/09/2004 Technical Basis Document for the Mound Site – Occupational Revision No.: 00 Controlled Copy No.: ________ Internal Dosimetry Page 1 of 65 Subject Expert: Jere Millard Supersedes: Document Owner Approval: Signature on File Date: 09/09/2004 Jeffrey S. Vollmer, TBD Team Leader None Approval: Signature on File Date: 09/09/2004 Judson L. Kenoyer, Task 3 Manager Concurrence: Signature on File Date: 09/09/2004 Richard E. Toohey, Project Director Approval: Signature on File Date: 09/09/2004 James W. Neton, Associate Director for Science TABLE OF CONTENTS Section Page Record of Issue/Revisions.................................................................................................................4 Acronyms and Abbreviations .............................................................................................................5 5.1 Introduction ..............................................................................................................................7 5.1.1 Mound Feed Materials .....................................................................................................7 5.1.2 Radionuclides of Concern ................................................................................................7 5.1.3 Internal Dose Control Program .........................................................................................9 5.1.4 Gross Alpha Analysis .................................................................................................... -

What Is Plutonium?
The Nuclear Regulatory Commission’s Science 101: What is Plutonium? Plutonium is a radioactive, metallic element with the atomic number 94. It was discovered in 1940 by scientists studying the process of splitting atoms. Plutonium is created in a nuclear reactor when uranium atoms, specifically uranium-238, absorb neutrons. Nearly all plutonium is man-made. Plutonium predominantly emits alpha particles—a type of radiation that does not penetrate and has a short range. It also emits neutrons, beta particles and gamma rays. It is considered toxic, in part, because if it were to be inhaled it could deposit in lungs and eventually cause damage to the tissue. Plutonium has five “common” isotopes, Pu-238, Pu-239, Pu-240, Pu-241, and Pu-242. All of the more common isotopes of plutonium are “fissionable”—which means the atom’s nucleus can easily split apart if it is struck by a neutron. The various isotopes of plutonium have been used in a number of applications. Plutonium-239 contains the highest quantities of fissile material, and is notably one of the primary fuels used in nuclear weapons. Plutonium-238 has more benign applications and has been used to power batteries for some heart pacemakers, as well as provide a long-lived heat source to power NASA space missions. Like uranium, plutonium can also be used to fuel nuclear power plants, as is done in a few countries. Currently, the U.S. does not use plutonium fuel in its power reactors. Nuclear reactors that produce commercial power in the United States today create plutonium through the irradiation of uranium fuel. -

Los Alamos NATIONAL LABORATORY
— . .. ,. ~ - /3~5Y” m5 ~“3 CIC-14 REPORT CQUECTW REPRODUCTION COPY Measurement and Accounting of the Minor Adinides Produced in Nuclear Power Reactors Los Alamos NATIONAL LABORATORY .i,os Alarnos National Laboratory is operated by the University of Cal~ornia for the United States Department of Energy under contract W-7405-ENG-36. Etlifeci by Paul IV. Fknriksen, Group ClC-l Prepared by Celirza M. CMz, Group lVIS-5 This work was supported by the U.S. Department of Energy, Ofice of lVonprol~eration and National Security, Ofice of Safeguards and Security, An Ajirmativc AcfionfEqual Opporfunify Employer This report waspreparedasan accountof worksponsoredby an agencyof theUnited States Govemrnent. NeitherTheRegentsof fhe Universityof Cal@rnia,the United Stafes Government norany agencythereof,norany of theiremployees,makesany warranty,express or implied,or assumesany legalliabilityor responsibilify~ortheaccuracy,completeness, or usefulness of any information, apparatus, product, or process disclosed,orrepresentsthat its use wouldnof infringe privately owned rights. Referenceherein to any speczfic commercial product, process, or seru”ce by trade name, trademark, manufacturer, or otherwise, doesnot necessarily constitute or imply its endorsement, recommendation, or favoring by The Regents of the University of California, fhe LInifedStates Government, or any agency thereof. The views and opinions of aufhors expressed herein do nof necessarily sfate or r~ect those of The Regents of the University of Calt@nia, the Unifed Sfates Government, or any agency thereo$ The Los A[amos National Laboratory strongly supports academic freedom and a researcher’s right to publish; therefore, the .!..aboratory as an institution doesnot endorse the viewpoint of a publication or guarantee its technical correctness. LA-13054-MS UC-700 Issued: January 1996 Measurement and Accounting of the Minor Actinides Produced in Nuclear Power Reactors J.