Problem Set 3 Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Two-Proton Radioactivity 2
Two-proton radioactivity Bertram Blank ‡ and Marek P loszajczak † ‡ Centre d’Etudes Nucl´eaires de Bordeaux-Gradignan - Universit´eBordeaux I - CNRS/IN2P3, Chemin du Solarium, B.P. 120, 33175 Gradignan Cedex, France † Grand Acc´el´erateur National d’Ions Lourds (GANIL), CEA/DSM-CNRS/IN2P3, BP 55027, 14076 Caen Cedex 05, France Abstract. In the first part of this review, experimental results which lead to the discovery of two-proton radioactivity are examined. Beyond two-proton emission from nuclear ground states, we also discuss experimental studies of two-proton emission from excited states populated either by nuclear β decay or by inelastic reactions. In the second part, we review the modern theory of two-proton radioactivity. An outlook to future experimental studies and theoretical developments will conclude this review. PACS numbers: 23.50.+z, 21.10.Tg, 21.60.-n, 24.10.-i Submitted to: Rep. Prog. Phys. Version: 17 December 2013 arXiv:0709.3797v2 [nucl-ex] 23 Apr 2008 Two-proton radioactivity 2 1. Introduction Atomic nuclei are made of two distinct particles, the protons and the neutrons. These nucleons constitute more than 99.95% of the mass of an atom. In order to form a stable atomic nucleus, a subtle equilibrium between the number of protons and neutrons has to be respected. This condition is fulfilled for 259 different combinations of protons and neutrons. These nuclei can be found on Earth. In addition, 26 nuclei form a quasi stable configuration, i.e. they decay with a half-life comparable or longer than the age of the Earth and are therefore still present on Earth. -

2.3 Neutrino-Less Double Electron Capture - Potential Tool to Determine the Majorana Neutrino Mass by Z.Sujkowski, S Wycech
DEPARTMENT OF NUCLEAR SPECTROSCOPY AND TECHNIQUE 39 The above conservatively large systematic hypothesis. TIle quoted uncertainties will be soon uncertainty reflects the fact that we did not finish reduced as our analysis progresses. evaluating the corrections fully in the current analysis We are simultaneously recording a large set of at the time of this writing, a situation that will soon radiative decay events for the processes t e'v y change. This result is to be compared with 1he and pi-+eN v y. The former will be used to extract previous most accurate measurement of McFarlane the ratio FA/Fv of the axial and vector form factors, a et al. (Phys. Rev. D 1984): quantity of great and longstanding interest to low BR = (1.026 ± 0.039)'1 I 0 energy effective QCD theory. Both processes are as well as with the Standard Model (SM) furthermore very sensitive to non- (V-A) admixtures in prediction (Particle Data Group - PDG 2000): the electroweak lagLangian, and thus can reveal BR = (I 038 - 1.041 )*1 0-s (90%C.L.) information on physics beyond the SM. We are currently analyzing these data and expect results soon. (1.005 - 1.008)* 1W') - excl. rad. corr. Tale 1 We see that even working result strongly confirms Current P1IBETA event sxpelilnentstatistics, compared with the the validity of the radiative corrections. Another world data set. interesting comparison is with the prediction based on Decay PIBETA World data set the most accurate evaluation of the CKM matrix n >60k 1.77k element V d based on the CVC hypothesis and ihce >60 1.77_ _ _ results -

22.101 Applied Nuclear Physics (Fall 2006) Problem Set No. 1 Due: Sept
22.101 Applied Nuclear Physics (Fall 2006) Problem Set No. 1 Due: Sept. 13, 2006 Problem 1 Before getting into the concepts of nuclear physics, every student should have some feeling for the numerical values of properties of nuclear radiations, such as energy and speed, wavelength, and frequency, etc. This involves some back of the envelope calculations using appropriate universal constants. (i) A thermal neutron in a nuclear reactor is a neutron with kinetic energy equal to kBT, where kB is the Boltzmann’s constant and T is the temperature of the reactor. Explain briefly the physical basis of this statement. Taking T to be the room temperature, 20C, calculate the energy of the thermal neutron (in units of ev), and then find its speed v (in cm/sec), the de Broglie wavelength λ (in A) and circular frequency ω (radian/sec). Compare these values with the energy, speed, and interatomic distance of atoms that make up the materials in the reactor. What is the point of comparing the neutron wavelength with typical atomic separations in a solid? (ii) Consider a 2 Kev x-ray, calculate the frequency and wavelength of this photon. What would be the point of comparing the x-ray wavelength with that of the thermal neutron? For an electron with wavelength equal to that of the thermal neutron, what energy would it have? 2 2 (iii) The classical radius of the electron, defined as e / mec , with e being the electron charge, me the electron rest mass, and c the speed of light, has the value of 2.818 x 10-13 cm. -

Abundance and Radioactivity of Unstable Isotopes
5 RADIONUCLIDE DECAY AND PRODUCTION This section contains a brief review of relevant facts about radioactivity, i.e. the phenomenon of nuclear decay, and about nuclear reactions, the production of nuclides. For full details the reader is referred to textbooks on nuclear physics and radiochemistry, and on the application of radioactivity in the earth sciences (cited in the list of references). 5.1 NUCLEAR INSTABILITY If a nucleus contains too great an excess of neutrons or protons, it will sooner or later disintegrate to form a stable nucleus. The different modes of radioactive decay will be discussed briefly. The various changes that can take place in the nucleus are shown in Fig.5.1 In transforming into a more stable nucleus, the unstable nucleus looses potential energy, part of its binding energy. This energy Q is emitted as kinetic energy and is shared by the particles formed according to the laws of conservation of energy and momentum (see Sect.5.3). The energy released during nuclear decay can be calculated from the mass budget of the decay reaction, using the equivalence of mass and energy, discussed in Sect.2.4. The atomic mass determinations have been made very accurately and precisely by mass spectrometric measurement: 2 E = [Mparent Mdaughter] P mc (5.1) with 1 amu V 931.5 MeV (5.2) Disintegration of a parent nucleus produces a daughter nucleus which generally is in an excited state. After an extremely short time the daughter nucleus looses the excitation energy through the emittance of one or more (gamma) rays, electromagnetic radiation with a very short wavelength, similar to the emission of light (also electromagnetic radiation , but with a longer wavelength) by atoms which have been brought into an excited state. -

Two-Proton Decay Energy and Width of 19Mg(G.S.)
University of Pennsylvania ScholarlyCommons Department of Physics Papers Department of Physics 7-30-2007 Two-Proton Decay Energy and Width of 19Mg(g.s.) H. Terry Fortune University of Pennsylvania, [email protected] Rubby Sherr Princeton University Follow this and additional works at: https://repository.upenn.edu/physics_papers Part of the Physics Commons Recommended Citation Fortune, H. T., & Sherr, R. (2007). Two-Proton Decay Energy and Width of 19Mg(g.s.). Retrieved from https://repository.upenn.edu/physics_papers/171 Suggested Citation: H.T. Fortune and R. Sherr. (2007). Two-proton decay energy and width of 19Mg(g.s.). Physical Review C 76, 014313. © 2007 The American Physical Society http://dx.doi.org/10.1103/PhysRevC.76.014313 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/physics_papers/171 For more information, please contact [email protected]. Two-Proton Decay Energy and Width of 19Mg(g.s.) Abstract We use a weak-coupling procedure and results of a shell-model calculation to compute the two-proton separation energy of 19Mg. Our result is at the upper end of the previous range, but 19Mg is still bound for single proton decay to 18Na. We also calculate the 2p decay width. Disciplines Physical Sciences and Mathematics | Physics Comments Suggested Citation: H.T. Fortune and R. Sherr. (2007). Two-proton decay energy and width of 19Mg(g.s.). Physical Review C 76, 014313. © 2007 The American Physical Society http://dx.doi.org/10.1103/PhysRevC.76.014313 This journal article is available at ScholarlyCommons: https://repository.upenn.edu/physics_papers/171 PHYSICAL REVIEW C 76, 014313 (2007) Two-proton decay energy and width of 19Mg(g.s.) H. -

CHAPTER 2 the Nucleus and Radioactive Decay
1 CHEMISTRY OF THE EARTH CHAPTER 2 The nucleus and radioactive decay 2.1 The atom and its nucleus An atom is characterized by the total positive charge in its nucleus and the atom’s mass. The positive charge in the nucleus is Ze , where Z is the total number of protons in the nucleus and e is the charge of one proton. The number of protons in an atom, Z, is known as the atomic number and dictates which element an atom represents. The nucleus is also made up of N number of neutrally charged particles of similar mass as the protons. These are called neutrons . The combined number of protons and neutrons, Z+N , is called the atomic mass number A. A specific nuclear species, or nuclide , is denoted by A Z Γ 2.1 where Γ represents the element’s symbol. The subscript Z is often dropped because it is redundant if the element’s symbol is also used. We will soon learn that a mole of protons and a mole of neutrons each have a mass of approximately 1 g, and therefore, the mass of a mole of Z+N should be very close to an integer 1. However, if we look at a periodic table, we will notice that an element’s atomic weight, which is the mass of one mole of its atoms, is rarely close to an integer. For example, Iridium’s atomic weight is 192.22 g/mole. The reason for this discrepancy is that an element’s neutron number N can vary. -

Module01 Nuclear Physics and Reactor Theory
Module I Nuclear physics and reactor theory International Atomic Energy Agency, May 2015 v1.0 Background In 1991, the General Conference (GC) in its resolution RES/552 requested the Director General to prepare 'a comprehensive proposal for education and training in both radiation protection and in nuclear safety' for consideration by the following GC in 1992. In 1992, the proposal was made by the Secretariat and after considering this proposal the General Conference requested the Director General to prepare a report on a possible programme of activities on education and training in radiological protection and nuclear safety in its resolution RES1584. In response to this request and as a first step, the Secretariat prepared a Standard Syllabus for the Post- graduate Educational Course in Radiation Protection. Subsequently, planning of specialised training courses and workshops in different areas of Standard Syllabus were also made. A similar approach was taken to develop basic professional training in nuclear safety. In January 1997, Programme Performance Assessment System (PPAS) recommended the preparation of a standard syllabus for nuclear safety based on Agency Safely Standard Series Documents and any other internationally accepted practices. A draft Standard Syllabus for Basic Professional Training Course in Nuclear Safety (BPTC) was prepared by a group of consultants in November 1997 and the syllabus was finalised in July 1998 in the second consultants meeting. The Basic Professional Training Course on Nuclear Safety was offered for the first time at the end of 1999, in English, in Saclay, France, in cooperation with Institut National des Sciences et Techniques Nucleaires/Commissariat a l'Energie Atomique (INSTN/CEA). -

RIA, Beta Decay and the R-Process
Preliminaries So Far Future Beta Decay, the R Process, RIA J. Engel April 4, 2006 Preliminaries So Far Future 1 Beta Decay is Important, Hard 2 What’s Been Done So Far 3 Future Work Preliminaries So Far Future Beta-Decay Far From Stability is Important 126 Limits of nuclear existence Half-lives are particularly 82 important at the r-process r-process “ladders” (N=50, 82, 126) 50 protons 82 ry where abundances peak. These d 28 rp-process 20 ctional Theo half-lives determine the 50 t Mean Fiel 8 28 neutrons Density Fun r-process time scale. 2 20 Selfconsisten 2 8 A=10 A~60 A=12 But others are also important; 0Ñω Shell Towards a unified affect abundances between Ab initio Model description of the nucleus few-body peaks. calculations No-Core Shell Model G-matrix Yes, uncertainties in astrophysics dwarf those in nuclear physics, but we won’t really understand the nucleosynthesis until both are reduced. 5 Preliminaries So Far Future Calculating Beta Decay is Hard. (though it gets easier in neutron-rich nuclei). To calculate beta decay between two states, you need: an accurate value for the decay energy ∆E (since −5 T1/2 ∝ ∆E ) matrix elements of the GT operator ~στ− and (sometimes) “forbidden” operators ~r~στ− between the two states .[Most of the strength of is above threshold, btw]. So your nuclear structure model must do a good job with nuclear masses, spectra, and wave functions, and to simulate the r process it must do the job in almost all isotopes. -

Enhancement Mechanisms of Low Energy Nuclear Reactions
Enhancement Mechanisms of Low Energy Nuclear Reactions Gareev F.A., Zhidkova I.E. Joint Institute for Nuclear Research, 141980, Dubna, Russia [email protected] [email protected] 1 Introduction One of the fundamental presentations of nuclear physics since the very early days of its study has been the common assumption that the radioactive process (the half-life or decay constant) is independent of external conditions. Rutherford, Chadwick and Ellis [1] came to the conclusion that: • ≪the value of λ (the decay constant) for any substance is a characteristic constant inde- pendent of all physical and chemical conditions≫. This very important conclusion (still playing a negative role in cold fusion phenomenon) is based on the common expectation (P. Curie suggested that the decay constant is the etalon of time) and observation that the radioactivity is a nuclear phenomenon since all our actions affect only states of the atom but do not change the nucleus states. We cannot hope to mention even a small part of the work done to establish the constancy of nuclear decay rates. For example, Emery G.T. stated [2]: • ≪Early workers tried to change the decay constants of various members of the natural radioactive series by varying the temperature between 24◦K and 1280◦K, by applying pressure of up to 2000 atm, by taking sources down into mines and up to the Jungfraujoch, by applying magnetic fields of up to 83,000 Gauss, by whirling sources in centrifuges, and by many other ingenious techniques. Occasional positive results were usually understood, arXiv:nucl-th/0505021v1 8 May 2005 in time, as result of changes in the counting geometry, or of the loss of volatile members of the natural decay chains. -

Phys586-Lec01-Radioa
Introduction ¾A more general title for this course might be “Radiation Detector Physics” ¾Goals are to understand the physics, detection, and applications of ionizing radiation The emphasis for this course is on radiation detection and applications to radiological physics However there is much overlap with experimental astro-, particle and nuclear physics And examples will be drawn from all of these fields 1 Introduction ¾While particle and medical radiation physics may seem unrelated, there is much commonality Interactions of radiation with matter is the same Detection principals of radiation are the same Some detectors are also the same, though possibly in different guises ¾Advances in medical physics have often followed quickly from advances in particle physics 2 Introduction ¾ Roentgen discovered x-rays in 1895 (Nobel Prize in 1901) ¾ A few weeks later he was photographing his wife’s hand ¾ Less than a year later x-rays were becoming routine in diagnostic radiography in US, Europe, and Japan ¾ Today the applications are ubiquitous (CAT, angiography, fluoroscopy, …) 3 Introduction ¾ Ernest Lawrence invented the cyclotron accelerator in 1930 (Nobel Prize in 1939) ¾ Five years later, John Lawrence began studies on cancer treatment using radioisotopes and neutrons (produced with the cyclotron) ¾ Their mother saved from cancer using massive x- ray dose 4 Introduction ¾Importance and relevance Radiation is often the only observable available in processes that occur on very short, very small, or very large scales Radiation detection -
Modern Physics to Which He Did This Observation, Usually to Within About 0.1%
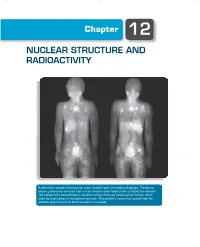
Chapter 12 NUCLEAR STRUCTURE AND RADIOACTIVITY Radioactive isotopes have proven to be valuable tools for medical diagnosis. The photo shows gamma-ray emission from a man who has been treated with a radioactive element. The radioactivity concentrates in locations where there are active cancer tumors, which show as bright areas in the gamma-ray scan. This patient’s cancer has spread from his prostate gland to several other locations in his body. 370 Chapter 12 | Nuclear Structure and Radioactivity The nucleus lies at the center of the atom, occupying only 10−15 of its volume but providing the electrical force that holds the atom together. Within the nucleus there are Z positive charges. To keep these charges from flying apart, the nuclear force must supply an attraction that overcomes their electrical repulsion. This nuclear force is the strongest of the known forces; it provides nuclear binding energies that are millions of times stronger than atomic binding energies. There are many similarities between atomic structure and nuclear structure, which will make our study of the properties of the nucleus somewhat easier. Nuclei are subject to the laws of quantum physics. They have ground and excited states and emit photons in transitions between the excited states. Just like atomic states, nuclear states can be labeled by their angular momentum. There are, however, two major differences between the study of atomic and nuclear properties. In atomic physics, the electrons experience the force provided by an external agent, the nucleus; in nuclear physics, there is no such external agent. In contrast to atomic physics, in which we can often consider the interactions among the electrons as a perturbation to the primary interaction between electrons and nucleus, in nuclear physics the mutual interaction of the nuclear constituents is just what provides the nuclear force, so we cannot treat this complicated many- body problem as a correction to a single-body problem.