Module01 Nuclear Physics and Reactor Theory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -

Models of the Atomic Nucleus
Models of the Atomic Nucleus Second Edition Norman D. Cook Models of the Atomic Nucleus Unification Through a Lattice of Nucleons Second Edition 123 Prof. Norman D. Cook Kansai University Dept. Informatics 569 Takatsuki, Osaka Japan [email protected] Additional material to this book can be downloaded from http://extras.springer.com. ISBN 978-3-642-14736-4 e-ISBN 978-3-642-14737-1 DOI 10.1007/978-3-642-14737-1 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2010936431 © Springer-Verlag Berlin Heidelberg 2006, 2010 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Cover design: WMXDesign GmbH, Heidelberg Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Preface to the Second Edition Already by the 1970s, some theorists had declared that nuclear structure physics was a “closed chapter” in science, but since then it has repeatedly been found necessary to re-open this closed chapter to address old problems and to explain new phenom- ena. -
Pair Energy of Proton and Neutron in Atomic Nuclei
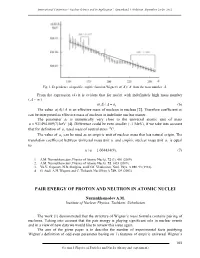
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

Compilation and Evaluation of Fission Yield Nuclear Data Iaea, Vienna, 2000 Iaea-Tecdoc-1168 Issn 1011–4289
IAEA-TECDOC-1168 Compilation and evaluation of fission yield nuclear data Final report of a co-ordinated research project 1991–1996 December 2000 The originating Section of this publication in the IAEA was: Nuclear Data Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria COMPILATION AND EVALUATION OF FISSION YIELD NUCLEAR DATA IAEA, VIENNA, 2000 IAEA-TECDOC-1168 ISSN 1011–4289 © IAEA, 2000 Printed by the IAEA in Austria December 2000 FOREWORD Fission product yields are required at several stages of the nuclear fuel cycle and are therefore included in all large international data files for reactor calculations and related applications. Such files are maintained and disseminated by the Nuclear Data Section of the IAEA as a member of an international data centres network. Users of these data are from the fields of reactor design and operation, waste management and nuclear materials safeguards, all of which are essential parts of the IAEA programme. In the 1980s, the number of measured fission yields increased so drastically that the manpower available for evaluating them to meet specific user needs was insufficient. To cope with this task, it was concluded in several meetings on fission product nuclear data, some of them convened by the IAEA, that international co-operation was required, and an IAEA co-ordinated research project (CRP) was recommended. This recommendation was endorsed by the International Nuclear Data Committee, an advisory body for the nuclear data programme of the IAEA. As a consequence, the CRP on the Compilation and Evaluation of Fission Yield Nuclear Data was initiated in 1991, after its scope, objectives and tasks had been defined by a preparatory meeting. -

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -

Appendix: Key Concepts and Vocabulary for Nuclear Energy
Appendix: Key Concepts and Vocabulary for Nuclear Energy The likelihood of fission depends on, among other Power Plant things, the energy of the incoming neutron. Some In most power plants around the world, heat, usually nuclei can undergo fission even when hit by a low- produced in the form of steam, is converted to energy neutron. Such elements are called fissile. electricity. The heat could come through the burning The most important fissile nuclides are the uranium of coal or natural gas, in the case of fossil-fueled isotopes, uranium-235 and uranium-233, and the power plants, or the fission of uranium or plutonium plutonium isotope, plutonium-239. Isotopes are nuclei. The rate of electrical power production in variants of the same chemical element that have these power plants is usually measured in megawatts the same number of protons and electrons, but or millions of watts, and a typical large coal or nuclear differ in the number of neutrons. Of these, only power plant today produces electricity at a rate of uranium-235 is found in nature, and it is found about 1,000 megawatts. A much smaller physical only in very low concentrations. Uranium in nature unit, the kilowatt, is a thousand watts, and large contains 0.7 percent uranium-235 and 99.3 percent household appliances use electricity at a rate of a few uranium-238. This more abundant variety is an kilowatts when they are running. The reader will have important example of a nucleus that can be split only heard about the “kilowatt-hour,” which is the amount by a high-energy neutron. -

22.101 Applied Nuclear Physics (Fall 2006) Problem Set No. 1 Due: Sept
22.101 Applied Nuclear Physics (Fall 2006) Problem Set No. 1 Due: Sept. 13, 2006 Problem 1 Before getting into the concepts of nuclear physics, every student should have some feeling for the numerical values of properties of nuclear radiations, such as energy and speed, wavelength, and frequency, etc. This involves some back of the envelope calculations using appropriate universal constants. (i) A thermal neutron in a nuclear reactor is a neutron with kinetic energy equal to kBT, where kB is the Boltzmann’s constant and T is the temperature of the reactor. Explain briefly the physical basis of this statement. Taking T to be the room temperature, 20C, calculate the energy of the thermal neutron (in units of ev), and then find its speed v (in cm/sec), the de Broglie wavelength λ (in A) and circular frequency ω (radian/sec). Compare these values with the energy, speed, and interatomic distance of atoms that make up the materials in the reactor. What is the point of comparing the neutron wavelength with typical atomic separations in a solid? (ii) Consider a 2 Kev x-ray, calculate the frequency and wavelength of this photon. What would be the point of comparing the x-ray wavelength with that of the thermal neutron? For an electron with wavelength equal to that of the thermal neutron, what energy would it have? 2 2 (iii) The classical radius of the electron, defined as e / mec , with e being the electron charge, me the electron rest mass, and c the speed of light, has the value of 2.818 x 10-13 cm. -

Global Fissile Material Report 2006 a Table of Contents
IPF M Global Fis sile Material Report Developing the technical basis for policy initiatives to secure and irreversibly reduce stocks of nuclear weapons and fissile materials 2006 Over the past six decades, our understanding of the nuclear danger has expanded from the threat posed by the vast nuclear arsenals created by the super- powers in the Cold War to encompass the prolifera- tion of nuclear weapons to additional states and now also to terrorist groups. To reduce this danger, it is essential to secure and to sharply reduce all stocks of highly enriched uranium and separated plutonium, the key materials in nuclear weapons, and to limit any further production. The mission of the IPFM is to advance the technical basis for cooperative international policy initiatives to achieve these goals. A report published by Global Fissile The International Panel on Fissile Materials (IPFM) www.fissilematerials.org Program on Science and Global Security Princeton University Material Report 2006 221 Nassau Street, 2nd Floor Princeton, NJ 08542, USA First report of the International Panel on Fissile Materials First report of the International Panel on Fissile Materials Developing the Technical Basis for Policy Initiatives to Secure and Irreversibly Reduce Stocks of Nuclear Weapons and Fissile Materials www.fissilematerials.org Global Fissile Material Report 2006 a Table of Contents About the IPFM 1 Summary 2 I. Background 5 1 Fissile Materials and Nuclear Weapons 6 2 Nuclear-Weapon and Fissile-Material Stocks 12 3 Production and Disposition of Fissile -

Prospects for Measurements with Strange Hadrons at Lhcb
Prospects for measurements with strange hadrons at LHCb A. A. Alves Junior1, M. O. Bettler2, A. Brea Rodr´ıguez1, A. Casais Vidal1, V. Chobanova1, X. Cid Vidal1, A. Contu3, G. D'Ambrosio4, J. Dalseno1, F. Dettori5, V.V. Gligorov6, G. Graziani7, D. Guadagnoli8, T. Kitahara9;10, C. Lazzeroni11, M. Lucio Mart´ınez1, M. Moulson12, C. Mar´ınBenito13, J. Mart´ınCamalich14;15, D. Mart´ınezSantos1, J. Prisciandaro 1, A. Puig Navarro16, M. Ramos Pernas1, V. Renaudin13, A. Sergi11, K. A. Zarebski11 1Instituto Galego de F´ısica de Altas Enerx´ıas(IGFAE), Santiago de Compostela, Spain 2Cavendish Laboratory, University of Cambridge, Cambridge, United Kingdom 3INFN Sezione di Cagliari, Cagliari, Italy 4INFN Sezione di Napoli, Napoli, Italy 5Oliver Lodge Laboratory, University of Liverpool, Liverpool, United Kingdom, now at Universit`adegli Studi di Cagliari, Cagliari, Italy 6LPNHE, Sorbonne Universit´e,Universit´eParis Diderot, CNRS/IN2P3, Paris, France 7INFN Sezione di Firenze, Firenze, Italy 8Laboratoire d'Annecy-le-Vieux de Physique Th´eorique , Annecy Cedex, France 9Institute for Theoretical Particle Physics (TTP), Karlsruhe Institute of Technology, Kalsruhe, Germany 10Institute for Nuclear Physics (IKP), Karlsruhe Institute of Technology, Kalsruhe, Germany 11School of Physics and Astronomy, University of Birmingham, Birmingham, United Kingdom 12INFN Laboratori Nazionali di Frascati, Frascati, Italy 13Laboratoire de l'Accelerateur Lineaire (LAL), Orsay, France 14Instituto de Astrof´ısica de Canarias and Universidad de La Laguna, Departamento de Astrof´ısica, La Laguna, Tenerife, Spain 15CERN, CH-1211, Geneva 23, Switzerland 16Physik-Institut, Universit¨atZ¨urich,Z¨urich,Switzerland arXiv:1808.03477v2 [hep-ex] 31 Jul 2019 Abstract This report details the capabilities of LHCb and its upgrades towards the study of kaons and hyperons. -

Problem Set 3 Solutions
22.01 Fall 2016, Problem Set 3 Solutions October 9, 2016 Complete all the assigned problems, and do make sure to show your intermediate work. 1 Activity and Half Lives 1. Given the half lives and modern-day abundances of the three natural isotopes of uranium, calculate the isotopic fractions of uranium when the Earth first formed 4.5 billion years ago. Today, uranium consists of 0.72% 235U, 99.2745% 238U, and 0.0055% 234U. However, it is clear that the half life of 234U (245,500 years) is so short compared to the lifetime of the Earth (4,500,000,000 years) that it would have all decayed away had there been some during the birth of the Earth. Therefore, we look a little closer, and find that 234U is an indirect decay product of 238U, by tracing it back from its parent nuclides on the KAERI table: α β− β− 238U −! 234T h −! 234P a −! 234U (1) Therefore we won’t consider there being any more 234U than would normally be in equi librium with the 238U around at the time. We set up the two remaining equations as follows: −t t ;235 −t t ;238 = 1=2 = 1=2 N235 = N0235 e N238 = N0238 e (2) Using the current isotopic abundances from above as N235 and N238 , the half lives from n 9 t 1 1 the KAERI Table of Nuclides t =2;235 = 703800000 y; t =2;238 = 4:468 · 10 y , and the lifetime of the earth in years (keeping everything in the same units), we arrive at the following expressions for N0235 and N0238 : N235 0:0072 N238 0:992745 N0235 =−t = 9 = 4:307N0238 =−t = 9 = 2:718 (3) =t1 ;235 −4:5·10 =7:038·108 =t1 ;238 −4:5·10 =4:468·109 e =2 e e =2 e Finally, taking the ratios of these two relative abundances gives us absolute abundances: 4:307 2:718 f235 = = 0:613 f238 = = 0:387 (4) 4:307 + 2:718 4:307 + 2:718 235U was 61.3% abundant, and 238U was 38.7% abundant. -

Nuclear Reactor Realities
Page 1 of 26 Nuclear Reactor Realities NUCLEAR REACTOR REALITIES (An Australian viewpoint) PREFACE Now that steam ships are no longer common, people tend to forget that nuclear power is just a replacement of coal, oil or gas for heating water to form steam to drive turbines, or of water (hydro) to do so directly. As will be shown in this tract, it is by far the safest way of doing so to generate electricity economically in large quantities – as a marine engineer of my acquaintance is fond of saying. Recently Quantum Market Research released its latest Australian Scan (The Advertiser, Saturday April 17, 2012, p 17). It has been tracking social change by interviewing 2000 Australians annually since 1992. In the concerns in the environment category, “[a]t the top of the list is nuclear accidents and waste disposal” (44.4 per cent), while “global warming” was well down the list of priorities at No 15, with only 27.7 per cent of people surveyed rating the issue as “extremely serious.” Part of the cause of such information must be that people are slowly realising that they have been deluded by publicity about unverified computer models which indicate that man’s emissions of CO2 play a major part in global warming. They have not yet realised that the history of the dangers of civilian nuclear power generation shows the reverse of their images. The topic of nuclear waste disposal is also shrouded in reactor physics mysteries, leading to a mis-placed general fear of the unknown. In this article only nuclear reactors are considered. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay.