Physics of Superheavy Elements Kouichi Hagino
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -
The Periodic Table of Elements
The Periodic Table of Elements 1 2 Atomic Number = Number of Protons = Number of Electrons H 6 He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo FRANCIUM RADIUM -

An Octad for Darmstadtium and Excitement for Copernicium
SYNOPSIS An Octad for Darmstadtium and Excitement for Copernicium The discovery that copernicium can decay into a new isotope of darmstadtium and the observation of a previously unseen excited state of copernicium provide clues to the location of the “island of stability.” By Katherine Wright holy grail of nuclear physics is to understand the stability uncover its position. of the periodic table’s heaviest elements. The problem Ais, these elements only exist in the lab and are hard to The team made their discoveries while studying the decay of make. In an experiment at the GSI Helmholtz Center for Heavy isotopes of flerovium, which they created by hitting a plutonium Ion Research in Germany, researchers have now observed a target with calcium ions. In their experiments, flerovium-288 previously unseen isotope of the heavy element darmstadtium (Z = 114, N = 174) decayed first into copernicium-284 and measured the decay of an excited state of an isotope of (Z = 112, N = 172) and then into darmstadtium-280 (Z = 110, another heavy element, copernicium [1]. The results could N = 170), a previously unseen isotope. They also measured an provide “anchor points” for theories that predict the stability of excited state of copernicium-282, another isotope of these heavy elements, says Anton Såmark-Roth, of Lund copernicium. Copernicium-282 is interesting because it University in Sweden, who helped conduct the experiments. contains an even number of protons and neutrons, and researchers had not previously measured an excited state of a A nuclide’s stability depends on how many protons (Z) and superheavy even-even nucleus, Såmark-Roth says. -

Darmstadtium, Roentgenium and Copernicium Form Strong Bonds with Cyanide
Darmstadtium, Roentgenium and Copernicium Form Strong Bonds With Cyanide Taye B. Demissie∗and Kenneth Ruudy March 23, 2017 Abstract We report the structures and properties of the cyanide complexes of three super- heavy elements (darmstadtium, roentgenium and copernicium) studied using two- and four-component relativistic methodologies. The electronic and structural properties of these complexes are compared to the corresponding complexes of platinum, gold and mercury. The results indicate that these superheavy elements form strong bonds with cyanide. Moreover, the calculated absorption spectra of these superheavy-element cyanides show similar trends to those of the corresponding heavy-atom cyanides. The calculated vibrational frequencies of the heavy-metal cyanides are in good agreement with available experimental results lending support to the quality of our calculated vibrational frequencies for the superheavy-atom cyanides. ∗Centre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic Uni- versity of Norway, N-9037 Tromsø, Norway yCentre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic Uni- versity of Norway, N-9037 Tromsø, Norway 1 Relativistic two- and four-component density-functional theory is used to demonstrate that the superheavy elements darmstadtium, roentgenium and copernicium form stable complexes with cyanide, providing new insight into the chemistry of these superheavy elements. 2 INTRODUCTION The term heavy atom refers roughly to elements in the 4th - -

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -

ROENTGENIUM Element Symbol: Rg Atomic Number: 111
ROENTGENIUM Element Symbol: Rg Atomic Number: 111 An initiative of IYC 2011 brought to you by the RACI PATRICIA ZUBER www.raci.org.au ROENTGENIUM Element symbol: Rg Atomic number: 111 Roentgenium has the symbol Rg and atomic number 111. However, it is not a stable element and can only be produced by cold fusion between other elements (for example by bombarding bismuth with nickel). Once formed, it is a synthetic radioactive element that falls apart in less than 20 sec. Therefore, there is currently no direct use for it. This element was first discovered by the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany in 1994. GSI also proposed a name for this new element: Roentgenium. They honoured the achievements of Wilhelm Conrad Röntgen, a physicist, who discovered X-rays in 1895 while being professor at the University of Wuerzburg in Germany. Röntgen is considered the father of diagnostic radiology, the medical specialty which uses imaging to diagnose disease. Roentgen never patented anything because he believed that knowledge should be available for everybody. He received the very first Nobel Prize in Physics in 1901. Provided by the element sponsor Martina Stenzel ARTISTS DESCRIPTION Lino cut, silk screen Roentgenium is a synthetic radioactive element produced by cold fusion. It has a half life of 20 seconds and decays by spontaneous fission. Not an easy element to create an image from! I decided to use a diagram of its atomic structure and fission lines as a basis for a relief image. I cut a paper stencil of Rg 111 and silk screened it in silver across the centre of this printed image. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

22.05 Reactor Physics – Part One Course Introduction
22.05 Reactor Physics – Part One Course Introduction 1. Instructor: John A. Bernard 2. Organization: Homework (20%) Four Exams (20% each; lowest grade is dropped) Final Exam (3.0 hours) (20%) 3. Text: The text book for this course is: Introduction to Nuclear Engineering, 3rd Edition, by John Lamarsh. This covers basic reactor physics as part of a complete survey of nuclear engineering. Readings may also be assigned from certain of the books listed below: Nuclear Reactor Analysis by A. F. Henry Introduction to Nuclear Power by G. Hewitt and J. Collier Fundamentals of Nuclear Science and Engineering by J. Shultis and R. Faw Atoms, Radiation, and Radiation Protection by J. Turner Nuclear Criticality Safety by R. Kneif Radiation Detection and Measurement by G. Knoll 4. Course Objective: To quote the late Professor Allan Henry: “The central problem of reactor physics can be stated quite simply. It is to compute, for any time t, the characteristics of the free-neutron population throughout an extended region of space containing an arbitrary, but known, mixture of materials. Specifically we wish to know the number of neutrons in any infinitesimal volume dV that have kinetic energies between E and E + ∆E and are traveling in directions within an infinitesimal angle of a fixed direction specified by the unit vector Ω. If this number is known, we can use the basic data obtained experimentally and theoretically from low-energy neutron physics to predict the rates at which all possible nuclear reactions, including fission, will take place throughout the region. Thus we can 1 predict how much nuclear power will be generated at any given time at any location in the region.” There are several reasons for needing this information: Physical understanding of reactor safety so that both design and operation is done intelligently. -
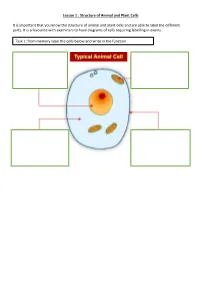
Lesson 1 : Structure of Animal and Plant Cells
Lesson 1 : Structure of Animal and Plant Cells It is important that you know the structure of animal and plant cells and are able to label the different parts. It is a favourite with examiners to have diagrams of cells requiring labelling in exams. Task 1: from memory label the cells below and write in the function Check your answers: There are many similarities and differences between animal and plant cells. Make sure you know these. Similarities Differences 1. Have a nucleus 1. Plant cells have a cellulose cell wall 2. Have a cytoplasm 2. Plant cells have a vacuole containing cell sap 3. Have a cell 3. Plant cells have chloroplast membrane 4. Contain 4. Many plant cells have a box-like shape whilst animal cell shape varies mitochondria 5. Plant cells have the nucleus to the side of the cell, animal cells have a nucleus in 5. Contain ribosomes the middle Task 2: Complete the sentences by filling in the gaps. Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. Check your answers: Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. -

Roentgenium Periodic Table of Elements
Periodic Table of Elements https://periodic-table.pro/Element/Rg/enView online at https://periodic-table.pro Roentgenium Roentgenium is named for Wilhelm Conrad Roentgen, who discovered x-rays. His element is highly radioactive, but disappointingly it does not, despite its name, emit x-rays when it decays. 01. OVERVIEW Symbol Rg Atomic number 111 Atomic weight 272 Density N/A Melting point N/A Boiling point N/A 02. THERMAL PROPERTIES Phase N/A Melting point N/A Boiling point N/A Absolute melting point N/A Absolute boiling point N/A Critical pressure N/A Critical temperature N/A Heat of fusion N/A Heat of vaporization N/A Heat of combustion N/A Specific heat N/A Adiabatic index N/A Neel point N/A Thermal conductivity N/A Thermal expansion N/A 03. PHYSICAL PROPERTIES Density N/A Density (liquid) N/A Molar volume N/A Molar mass 282.169u Brinell hardness N/A Mohs hardness N/A Vickers hardness N/A Bulk modulus N/A Shear modulus N/A Young modulus N/A Poisson ratio N/A Refractive index N/A Speed of sound N/A Thermal conductivity N/A Thermal expansion N/A 04. REACTIVITY Valence N/A Electronegativity N/A Electron affinity N/A Ionization energies N/A 05. SAFETY Autoignition point N/A Flashpoint N/A Heat of combustion N/A 06. CLASSIFICATIONS Alternate names N/A Names of allotropes N/A Block, Group, Period d, 11, 7 Electron configuration [Rn]5f¹⁴6d⁹7s² Color N/A Discovery 1994 in Germany Gas phase N/A 07. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Section 29-4: the Chart of the Nuclides
29-4 The Chart of the Nuclides A nuclide is an atom that is characterized by what is in its nucleus. In other words, it is characterized by the number of protons it has, and by the number of neutrons it has. Figure 29.2 shows the chart of the nuclides, which plots, for stable and radioactive nuclides, the value of Z (the atomic number), on the vertical axis, and the value of N (the number of neutrons) on the horizontal axis. If you look at a horizontal line through the chart, you will find nuclides that all have the same number of protons, but a different number of neutrons. These are all nuclides of the same element, and are known as isotopes (equal proton number, different neutron number). On a vertical line through the chart, the nuclides all have the same number of neutrons, but a different number of protons. These nuclides are known as isotones (equal neutron number, different proton number). It is interesting to think about how the various decay processes play a role in the chart of the nuclides. Only the nuclides shown in black are stable. The stable nuclides give the chart a line of stability that goes from the bottom left toward the upper right, curving down and away from the Z = N line as you move toward higher N values. There are many more nuclides that are shown on the chart but which are not stable - these nuclides decay by means of a radioactive decay process. In general, the dominant decay process for a particular nuclide is the process that produces a nucleus closer to the line of stability than where it started.