Isotonic and Isotopic Dependence of the Radiative Neutron Capture Cross-Section on the Neutron Excess
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neutron Interactions and Dosimetry Outline Introduction Tissue
Outline • Neutron dosimetry Neutron Interactions and – Thermal neutrons Dosimetry – Intermediate-energy neutrons – Fast neutrons Chapter 16 • Sources of neutrons • Mixed field dosimetry, paired dosimeters F.A. Attix, Introduction to Radiological • Rem meters Physics and Radiation Dosimetry Introduction Tissue composition • Consider neutron interactions with the majority tissue elements H, O, C, and N, and the resulting absorbed dose • Because of the short ranges of the secondary charged particles that are produced in such interactions, CPE is usually well approximated • Since no bremsstrahlung x-rays are generated, the • The ICRU composition for muscle has been assumed in absorbed dose can be assumed to be equal to the most cases for neutron-dose calculations, lumping the kerma at any point in neutron fields at least up to 1.1% of “other” minor elements together with oxygen to an energy E ~ 20 MeV make a simple four-element (H, O, C, N) composition Neutron kinetic energy Neutron kinetic energy • Neutron fields are divided into three • Thermal neutrons, by definition, have the most probable categories based on their kinetic energy: kinetic energy E=kT=0.025eV at T=20C – Thermal (E<0.5 eV) • Neutrons up to 0.5eV are considered “thermal” due to simplicity of experimental test after they emerge from – Intermediate-energy (0.5 eV<E<10 keV) moderator material – Fast (E>10 keV) • Cadmium ratio test: • Differ by their primary interactions in tissue – Gold foil can be activated through 197Au(n,)198Au interaction and resulting biological effects -

Concrete Analysis by Neutron-Capture Gamma Rays Using Californium 252
CONCRETE ANALYSIS BY NEUTRON-CAPTURE GAMMA RAYS USING CALIFORNIUM 252 Dick Duffey, College of Engineering, University of Maryland; Peter F. Wiggins, Naval Systems Engineering Department, U.S. Naval Academy; Frank E. Senftle, U.S. Geological Survey; and A. A. El Kady, United Arab Republic Atomic Energy Establishment, Cairo The feasibility of analyzing concrete and cement by a measurement of the neutron-capture or prompt gamma rays was investigated; a 100-ug californium-252 source was used to supply the neutrons. A lithium drifted germanium crystal detected the capture gamma rays emitted. The capture gamma rays from cement, sand, and 3 coarse aggregates-quartzite gravel, limestone, and diabase--:were studied. Concrete blocks made from these materials were then tested. The capture gamma ray response of the calcium, silicon, and iron in the concrete blocks was in accord with the elements identified in the mix materials. The principal spectral lines used were the 6.42 MeV line of calcium, the 4.93 MeV line of silicon, and the doublet of iron at about 7 .64 MeV. The aluminum line at 7. 72 MeV was ob served in some cases but at a lower intensity with the limited electronic equipment available. This nuclear spectroscopic technique offers a possi ble method of determining the components of sizable concrete samples in a nondestructive, in situ manner. In addition, the neutron-capture gamma ray technique might find application in control of the concrete and cement processes and furnish needed information on production operations and inventories. • FROM THE point of view of the geochemical analyst, concrete may be considered as rock relocated and reformed at the convenience of the engineer. -

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -

1663-29-Othernuclearreaction.Pdf
it’s not fission or fusion. It’s not alpha, beta, or gamma dosimeter around his neck to track his exposure to radiation decay, nor any other nuclear reaction normally discussed in in the lab. And when he’s not in the lab, he can keep tabs on his an introductory physics textbook. Yet it is responsible for various experiments simultaneously from his office computer the existence of more than two thirds of the elements on the with not one or two but five widescreen monitors—displaying periodic table and is virtually ubiquitous anywhere nuclear graphs and computer codes without a single pixel of unused reactions are taking place—in nuclear reactors, nuclear bombs, space. Data printouts pinned to the wall on the left side of the stellar cores, and supernova explosions. office and techno-scribble densely covering the whiteboard It’s neutron capture, in which a neutron merges with an on the right side testify to a man on a mission: developing, or atomic nucleus. And at first blush, it may even sound deserving at least contributing to, a detailed understanding of complex of its relative obscurity, since neutrons are electrically neutral. atomic nuclei. For that, he’ll need to collect and tabulate a lot For example, add a neutron to carbon’s most common isotope, of cold, hard data. carbon-12, and you just get carbon-13. It’s slightly heavier than Mosby’s primary experimental apparatus for doing this carbon-12, but in terms of how it looks and behaves, the two is the Detector for Advanced Neutron Capture Experiments are essentially identical. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

22.05 Reactor Physics – Part One Course Introduction
22.05 Reactor Physics – Part One Course Introduction 1. Instructor: John A. Bernard 2. Organization: Homework (20%) Four Exams (20% each; lowest grade is dropped) Final Exam (3.0 hours) (20%) 3. Text: The text book for this course is: Introduction to Nuclear Engineering, 3rd Edition, by John Lamarsh. This covers basic reactor physics as part of a complete survey of nuclear engineering. Readings may also be assigned from certain of the books listed below: Nuclear Reactor Analysis by A. F. Henry Introduction to Nuclear Power by G. Hewitt and J. Collier Fundamentals of Nuclear Science and Engineering by J. Shultis and R. Faw Atoms, Radiation, and Radiation Protection by J. Turner Nuclear Criticality Safety by R. Kneif Radiation Detection and Measurement by G. Knoll 4. Course Objective: To quote the late Professor Allan Henry: “The central problem of reactor physics can be stated quite simply. It is to compute, for any time t, the characteristics of the free-neutron population throughout an extended region of space containing an arbitrary, but known, mixture of materials. Specifically we wish to know the number of neutrons in any infinitesimal volume dV that have kinetic energies between E and E + ∆E and are traveling in directions within an infinitesimal angle of a fixed direction specified by the unit vector Ω. If this number is known, we can use the basic data obtained experimentally and theoretically from low-energy neutron physics to predict the rates at which all possible nuclear reactions, including fission, will take place throughout the region. Thus we can 1 predict how much nuclear power will be generated at any given time at any location in the region.” There are several reasons for needing this information: Physical understanding of reactor safety so that both design and operation is done intelligently. -

STUDY of the NEUTRON and PROTON CAPTURE REACTIONS 10,11B(N, ), 11B(P, ), 14C(P, ), and 15N(P, ) at THERMAL and ASTROPHYSICAL ENERGIES
STUDY OF THE NEUTRON AND PROTON CAPTURE REACTIONS 10,11B(n, ), 11B(p, ), 14C(p, ), AND 15N(p, ) AT THERMAL AND ASTROPHYSICAL ENERGIES SERGEY DUBOVICHENKO*,†, ALBERT DZHAZAIROV-KAKHRAMANOV*,† *V. G. Fessenkov Astrophysical Institute “NCSRT” NSA RK, 050020, Observatory 23, Kamenskoe plato, Almaty, Kazakhstan †Institute of Nuclear Physics CAE MINT RK, 050032, str. Ibragimova 1, Almaty, Kazakhstan *[email protected] †[email protected] We have studied the neutron-capture reactions 10,11B(n, ) and the role of the 11B(n, ) reaction in seeding r-process nucleosynthesis. The possibility of the description of the available experimental data for cross sections of the neutron capture reaction on 10B at thermal and astrophysical energies, taking into account the resonance at 475 keV, was considered within the framework of the modified potential cluster model (MPCM) with forbidden states and accounting for the resonance behavior of the scattering phase shifts. In the framework of the same model the possibility of describing the available experimental data for the total cross sections of the neutron radiative capture on 11B at thermal and astrophysical energies were considered with taking into account the 21 and 430 keV resonances. Description of the available experimental data on the total cross sections and astrophysical S-factor of the radiative proton capture on 11B to the ground state of 12C was treated at astrophysical energies. The possibility of description of the experimental data for the astrophysical S-factor of the radiative proton capture on 14C to the ground state of 15N at astrophysical energies, and the radiative proton capture on 15N at the energies from 50 to 1500 keV was considered in the framework of the MPCM with the classification of the orbital states according to Young tableaux. -

Energy Deposition by Neutrons
22.55 “Principles of Radiation Interactions” Neutrons Classification of neutrons by energy Thermal: E < 1 eV (0.025 eV) Epithermal: 1 eV < E < 10 keV Fast: > 10 keV Neutron sources Neutron energies Reactors neutrons in the few keV to several MeV Fusion reactions 14 MeV Large accelerators Hundreds of MeV Energy Deposition by Neutrons • Neutrons are generated over a wide range of energies by a variety of different processes. • Like photons, neutrons are uncharged and do not interact with orbital electrons. • Neutrons can travel considerable distances through matter without interacting. • Neutrons will interact with atomic nuclei through several mechanisms. o Elastic scatter o Inelastic scatter o Nonelastic scatter o Neutron capture o Spallation • The type of interaction depends on the neutron energy Radiation Interactions: neutrons Page 1 of 8 22.55 “Principles of Radiation Interactions” Cross Sections • Because mass attenuation coefficients, µ/ρ (cm2/g) have dimensions of cm2 in the numerator, they have come to be called “cross sections”. • Cross sections do not represent a physical area, but a probability of an interaction. • Cross sections usually expressed in the unit, barn: (10-24 cm2) • The atomic cross sections can be derived from the mass attenuation coefficient. Photons Cross sections are attenuation coefficients, expressed at the atom level (Probability of interaction per atom) ρ N = atom density (#atoms/cm3) N = N A A A 0 2 σA = atomic cross section (cm /atom) 23 N0 = 6.02 x 10 atoms/mole ρ = g/cm3 µ = N A σ A A = g/mole ρ µ = N σ A 0 A µ N σ ⎛ µ ⎞⎛ A ⎞ 0 A ⎜ ⎟⎜ ⎟ = σ A = ⎜ ⎟⎜ ⎟ ρ A ⎝ ρ ⎠⎝ N 0 ⎠ Radiation Interactions: neutrons Page 2 of 8 22.55 “Principles of Radiation Interactions” Neutron Cross Sections Analogous to photons • Neutrons interact by different mechanisms depending on the neutron energy and the material of the absorber o Scattering • elastic • inelastic o Capture • Each energy loss mechanism has a cross section • Neutron cross sections expressed in barns (1 barn = 10-24 cm2). -
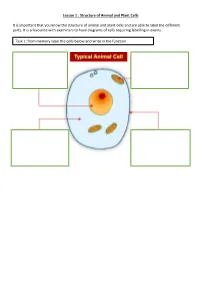
Lesson 1 : Structure of Animal and Plant Cells
Lesson 1 : Structure of Animal and Plant Cells It is important that you know the structure of animal and plant cells and are able to label the different parts. It is a favourite with examiners to have diagrams of cells requiring labelling in exams. Task 1: from memory label the cells below and write in the function Check your answers: There are many similarities and differences between animal and plant cells. Make sure you know these. Similarities Differences 1. Have a nucleus 1. Plant cells have a cellulose cell wall 2. Have a cytoplasm 2. Plant cells have a vacuole containing cell sap 3. Have a cell 3. Plant cells have chloroplast membrane 4. Contain 4. Many plant cells have a box-like shape whilst animal cell shape varies mitochondria 5. Plant cells have the nucleus to the side of the cell, animal cells have a nucleus in 5. Contain ribosomes the middle Task 2: Complete the sentences by filling in the gaps. Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. Check your answers: Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. -

Computing Neutron Capture Rates in Neutron-Degenerate Matter †
universe Article Computing Neutron Capture Rates in Neutron-Degenerate Matter † Bryn Knight and Liliana Caballero * Department of Physics, University of Guelph, Guelph, ON N1G 2W1, Canada; [email protected] * Correspondence: [email protected] † This paper is based on the talk at the 7th International Conference on New Frontiers in Physics (ICNFP 2018), Crete, Greece, 4–12 July 2018. Received: 28 November 2018; Accepted: 16 January 2019; Published: 18 January 2019 Abstract: Neutron captures are likely to occur in the crust of accreting neutron stars (NSs). Their rate depends on the thermodynamic state of neutrons in the crust. At high densities, neutrons are degenerate. We find degeneracy corrections to neutron capture rates off nuclei, using cross sections evaluated with the reaction code TALYS. We numerically integrate the relevant cross sections over the statistical distribution functions of neutrons at thermodynamic conditions present in the NS crust. We compare our results to analytical calculations of these corrections based on a power-law behavior of the cross section. We find that although an analytical integration can simplify the calculation and incorporation of the results for nucleosynthesis networks, there are uncertainties caused by departures of the cross section from the power-law approach at energies close to the neutron chemical potential. These deviations produce non-negligible corrections that can be important in the NS crust. Keywords: neutron capture; neutron stars; degenerate matter; nuclear reactions 1. Introduction X-ray burst and superburst observations are attributed to accreting neutron stars (NSs) (see, e.g., [1,2]). In such a scenario, a NS drags matter from a companion star. -

Neutron Capture Cross Sections of Cadmium Isotopes
Neutron Capture Cross Sections of Cadmium Isotopes By Allison Gicking A thesis submitted to Oregon State University In partial fulfillment of the requirements for the degree of Bachelor of Science Presented June 8, 2011 Commencement June 17, 2012 Abstract The neutron capture cross sections of 106Cd, 108Cd, 110Cd, 112Cd, 114Cd and 116Cd were determined in the present project. Four different OSU TRIGA reactor facilities were used to produce redundancy in the results and to measure the thermal cross section and resonance integral separately. When the present values were compared with previously measured values, the differences were mostly due to the kind of detector used or whether or not the samples were natural cadmium. Some of the isotopes did not have any previously measured values, and in that case, new information about the cross sections of those cadmium isotopes has been provided. Table of Contents I. Introduction………………………………………………………………….…….…1 II. Theory………………………………………………………………………...…...…3 1. Neutron Capture…………………………………………………….….……3 2. Resonance Integral vs. Effective Thermal Cross Section…………...………5 3. Derivation of the Activity Equations…………………………………....…..8 III. Methods………………………………………………………….................…...…...12 1. Irradiation of the Samples………………………………………….….....…12 2. Sample Preparation and Parameters………………..………...………..……16 3. Efficiency Calibration of Detectors…………………………..………....…..18 4. Data Analysis…………………………………...…….………………...…..19 5. Absorption by 113Cd……………………………………...……...….………20 IV. Results………………………………………………….……………..……….…….22 -

Physics of Superheavy Elements Kouichi Hagino
Frontiers in Science II 2013.11.6 Physics of superheavy elements Kouichi Hagino Nuclear Theory Group, Department of Physics, Tohoku University What is nuclear physics? What are superheavy elements? How to create superheavy elements? What are chemical properties of superheavy elements? Introduction: atoms and atomic nuclei What would you see if you magnified the dog? ~ 50 cm Introduction: atoms and atomic nuclei cells ~ 50 cm ~ m = 10-6 m Introduction: atoms and atomic nuclei DNA cells -8 ~ 50 cm ~ m = 10-6 m ~ 10 m atom All things are made of atoms. ~ 10-10 m All things are made of atoms. • Thales, Democritus (ancient Greek) • Dalton (chemist, 19th century) • Boltzmann(19th century) • Einstein (1905) ~ 10-10 m STM image (surface physics group, Tohoku university) Introduction: atoms and atomic nuclei DNA cells -8 ~ 50 cm ~ 10 m atom atomic nucleus ~ 10-15 m ~ 10-10 m proton (+e) neutron (no charge) electron cloud (-e) Neutral atoms: # of protons = # of electrons Chemical properties of atoms # of electrons Mp ~ Mn ~ 2000 Me the mass of atom ~ the mass of nucleus Periodic table of chemical elements tabular arrangement of chemical elements based on the atomic numbers (= # of electrons = # of protons) What are we made of ? oxygen 43 kg cerium 40 mg gallium 0.7 mg carbon 16 kg barium 22 mg tellurium 0.7 mg hydrogen 7 kg iodine 20 mg yttrium 0.6 mg nitrogen 1.8 kg tin 20 mg bismuth 0.5 mg calcium 1.0 kg titanium 20 mg thallium 0.5 mg phosphorus 780 g boron 18 mg indium 0.4 mg potassium 140 g nickel 15 mg gold 0.2 mg sulphur 140 g selenium