Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

NOBELIUM Element Symbol: No Atomic Number: 102
NOBELIUM Element Symbol: No Atomic Number: 102 An initiative of IYC 2011 brought to you by the RACI KERRY LAMB www.raci.org.au NOBELIUM Element symbol: No Atomic number: 102 The credit for discovering Nobelium was disputed with 3 different research teams claiming the discovery. While the first claim dates back to 1957, it was not until 1992 that the International Union of Pure and Applied Chemistry credited the discovery to a research team from Dubna in Russia for work they did in 1966. The element was named Nobelium in 1957 by the first of its claimed discoverers (the Nobel Institute in Sweden). It was named after Alfred Nobel, a Swedish chemist who invented dynamite, held more than 350 patents and bequeathed his fortune to the establishment of the Nobel Prizes. Nobelium is a synthetic element and does not occur in nature and has no known uses other than in scientific research as only tiny amounts of the element have ever been produced. Nobelium is radioactive and most likely metallic. The appearance and properties of Nobelium are unknown as insufficient amounts of the element have been produced. Nobelium is made by the bombardment of curium (Cm) with carbon nuclei. Its most stable isotope, 259No, has a half-life of 58 minutes and decays to Fermium (255Fm) through alpha decay or to Mendelevium (259Md) through electron capture. Provided by the element sponsor Freehills Patent and Trade Mark Attorneys ARTISTS DESCRIPTION I wanted to depict Alfred Nobel, the namesake of Nobelium, as a resolute young man, wearing the Laurel wreath which is the symbol of victory. -

CHEMISTRY for the Bottom of the Periodic Table
http://cyclotron.tamu.edu CHEMISTRY for the Bottom of the Periodic Table Techniques to investigate chemical properties of superheavy elements lead to improved methods for separating heavy metals THE SCIENCE The chemical properties of superheavy element 113, nihonium, are almost completely unknown, so a team of researchers from the Cyclotron Institute at Texas A&M University and the Institut Pluridisciplinaire Hubert Curien in France are developing techniques that could be used to study this fleeting element. As part of that effort, they are comparing the properties of nihonium to the chemically similar elements indium and thallium; to do so, the team studied the separation of these two elements using a new class of designer molecules called ionic liquids. THE IMPACT Measuring the chemical properties of nihonium and other superheavy elements will increase our understanding of the principles that control the Periodic Table. Comparing the data from nihonium to results for similar elements, obtained using the team’s fast, efficient, single-step process, reveals trends that arise from the structure of the Periodic Table. This research could also lead to better methods of re- using indium, a metal that is part of flat-panel displays but not currently mined in the United States. The proposed mechanism of transfer. Thallium (Tl) bonds with chlorine (Cl) and moves into the ionic liquid (in blue). SUMMARY The distribution of indium and thallium between the aque- ous and organic phases is the key to understanding the PUBLICATIONS separation of these elements. An aqueous solution, con- E.E. Tereshatov, M. Yu. Boltoeva, V. Mazan, M.F. -
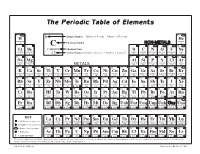
The Periodic Table of Elements
The Periodic Table of Elements 1 2 Atomic Number = Number of Protons = Number of Electrons H 6 He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo FRANCIUM RADIUM -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

IUPAC Wire See Also
News and information on IUPAC, its fellows, and member organizations. IUPAC Wire See also www.iupac.org Flerovium and Livermorium Join the Future Earth: Research for Global Periodic Table Sustainability n 30 May 2012, IUPAC officially approved he International Council for Science (ICSU), of the name flerovium, with symbol Fl, for the which IUPAC is a member, announced a new Oelement of atomic number 114 and the name T10-year initiative named Future Earth to unify livermorium, with symbol Lv, for the element of atomic and scale up ICSU-sponsored global environmental- number 116. The names and symbols were proposed change research. by the collaborating team of the Joint Institute for Nuclear Research (Dubna, Russia) and the Lawrence Operational in 2013, this new ICSU initiative will Livermore National Laboratory (Livermore, California, provide a cutting-edge platform to coordinate scien- USA) to whom the priority for the discovery of these tific research to respond to the most critical social and elements was assigned last year. The IUPAC recom- environmental challenges of the 21st century at global mendations presenting these names is to appear in and regional levels. “This initiative will link global envi- the July 2012 issue of Pure and Applied Chemistry. ronmental change and fundamental human develop- ment questions,” said Diana Liverman, co-director of The name flerovium, with symbol Fl, lies within the Institute of the Environment at the University of tradition and honors the Flerov Laboratory of Nuclear Arizona and co-chair of the team Reactions in Dubna, Russia, where the element of that is designing Future Earth. -

The Histories Hidden in the Periodic Table
The Histories Hidden in the Periodic Table From poisoned monks and nuclear bombs to the “transfermium wars,” mapping the atomic world hasn’t been easy. By Neima Jahromi 6:00 A.M. As element hunters have become element makers, the periodic table’s meaning has changed. It now describes what is possible, in addition to what merely exists. Illustration by Ilya Milstein The story of the fifteenth element began in Hamburg, in 1669. The unsuccessful glassblower and alchemist Hennig Brandt was trying to find the philosopher’s stone, a mythical substance that could turn base metals into gold. Instead, he distilled something new. It was foamy and, depending on the preparation, yellow or black. He called it “cold fire,” because it glowed in the dark. Interested parties took a look; some felt that they were in the presence of a miracle. “If anyone had rubbed himself all over with it,” one observer noted, “his whole figure would have shone, as once did that of Moses when he came down from Mt. Sinai.” Robert Boyle, the father of modern chemistry, put some on his hand and noted how “mild and innocent” it seemed. Another scientist saw particles in it twinkling “like little stars.” At first, no one could figure out what the Prometheus of Hamburg had stolen. After one of Brandt’s confidants provided a hint—the main ingredient was “somewhat that belong’d to the Body of Man”—Boyle deduced that he and his peers had been smearing themselves with processed urine. As the Cambridge chemist Peter Wothers explains in his new history of the elements, “Antimony, Gold, and Jupiter’s Wolf” (Oxford), Brandt’s recipe called for a ton of urine. -

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -
1 Chapter 16 Stable and Radioactive Isotopes Jim Murray 5/7/01 Univ
Chapter 16 Stable and Radioactive Isotopes Jim Murray 5/7/01 Univ. Washington Each atomic element (defined by its number of protons) comes in different flavors, depending on the number of neutrons. Most elements in the periodic table exist in more than one isotope. Some are stable and some are radioactive. Scientists have tallied more than 3600 isotopes, the majority are radioactive. The Isotopes Project at Lawrence Berkeley National Lab in California has a web site (http://ie.lbl.gov/toi.htm) that gives detailed information about all the isotopes. Stable and radioactive isotopes are the most useful class of tracers available to geochemists. In almost all cases the distributions of these isotopes have been used to study oceanographic processes controlling the distributions of the elements. Radioactive isotopes are especially useful because they provide a way to put time into geochemical models. The chemical characteristic of an element is determined by the number of protons in its nucleus. Different elements can have different numbers of neutrons and thus atomic weights (the sum of protons plus neutrons). The atomic weight is equal to the sum of protons plus neutrons. The chart of the nuclides (protons versus neutrons) for elements 1 (Hydrogen) through 12 (Magnesium) is shown in Fig. 16-1. The Valley of Stability represents nuclides stable relative to decay. Examples: Atomic Protons Neutrons % Abundance Weight (Atomic Number) Carbon 12C 6P 6N 98.89 13C 6P 7N 1.11 14C 6P 8N 10-10 Oxygen 16O 8P 8N 99.76 17O 8P 9N 0.024 18O 8P 10N 0.20 Several light elements such as H, C, N, O, and S have more than one stable isotope form, which show variable abundances in natural samples. -

22.05 Reactor Physics – Part One Course Introduction
22.05 Reactor Physics – Part One Course Introduction 1. Instructor: John A. Bernard 2. Organization: Homework (20%) Four Exams (20% each; lowest grade is dropped) Final Exam (3.0 hours) (20%) 3. Text: The text book for this course is: Introduction to Nuclear Engineering, 3rd Edition, by John Lamarsh. This covers basic reactor physics as part of a complete survey of nuclear engineering. Readings may also be assigned from certain of the books listed below: Nuclear Reactor Analysis by A. F. Henry Introduction to Nuclear Power by G. Hewitt and J. Collier Fundamentals of Nuclear Science and Engineering by J. Shultis and R. Faw Atoms, Radiation, and Radiation Protection by J. Turner Nuclear Criticality Safety by R. Kneif Radiation Detection and Measurement by G. Knoll 4. Course Objective: To quote the late Professor Allan Henry: “The central problem of reactor physics can be stated quite simply. It is to compute, for any time t, the characteristics of the free-neutron population throughout an extended region of space containing an arbitrary, but known, mixture of materials. Specifically we wish to know the number of neutrons in any infinitesimal volume dV that have kinetic energies between E and E + ∆E and are traveling in directions within an infinitesimal angle of a fixed direction specified by the unit vector Ω. If this number is known, we can use the basic data obtained experimentally and theoretically from low-energy neutron physics to predict the rates at which all possible nuclear reactions, including fission, will take place throughout the region. Thus we can 1 predict how much nuclear power will be generated at any given time at any location in the region.” There are several reasons for needing this information: Physical understanding of reactor safety so that both design and operation is done intelligently. -

Nuclear Binding & Stability
Nuclear Binding & Stability Stanley Yen TRIUMF UNITS: ENERGY Energy measured in electron-Volts (eV) 1 volt battery boosts energy of electrons by 1 eV 1 volt -19 battery 1 e-Volt = 1.6x10 Joule 1 MeV = 106 eV 1 GeV = 109 eV Recall that atomic and molecular energies ~ eV nuclear energies ~ MeV UNITS: MASS From E = mc2 m = E / c2 so we measure masses in MeV/c2 1 MeV/c2 = 1.7827 x 10 -30 kg Frequently, we get lazy and just set c=1, so that we measure masses in MeV e.g. mass of electron = 0.511 MeV mass of proton = 938.272 MeV mass of neutron=939.565 MeV Also widely used unit of mass is the atomic mass unit (amu or u) defined so that Mass(12C atom) = 12 u -27 1 u = 931.494 MeV = 1.6605 x 10 kg nucleon = proton or neutron “nuclide” means one particular nuclear species, e.g. 7Li and 56Fe are two different nuclides There are 4 fundamental types of forces in the universe. 1. Gravity – very weak, negligible for nuclei except for neutron stars 2. Electromagnetic forces – Coulomb repulsion tends to force protons apart ) 3. Strong nuclear force – binds nuclei together; short-ranged 4. Weak nuclear force – causes nuclear beta decay, almost negligible compared to the strong and EM forces. How tightly a nucleus is bound together is mostly an interplay between the attractive strong force and the repulsive electromagnetic force. Let's make a scatterplot of all the stable nuclei, with proton number Z versus neutron number N. -

Interactive Chemistry Exhibition
United Nations International Year All-Russian United Nations International Year All-Russian Educational, Scientific and of the Periodic Table Science Educational, Scientific and of the Periodic Table Science Cultural Organization of Chemical Elements Festival Cultural Organization of Chemical Elements Festival Science for All: Interactive Chemistry Exhibition The Management Committee of the International Year of the Periodic Table of Chemical Elements (IYPT2019) in partnership with All - Russian Science Festival invites you to visit an Interactive Exhibition on Chemistry at UNESCO Headquarters from 28 to 30 January 2019. Launched as part of the Opening Ceremony of the International Year of the Periodic Table of Chemical Elements (IYPT2019) on January 29th, this exhibition will travel around the world during the year 2019. Science for All: Interactive Chemistry Exhibition - an exciting journey into the world of «living» Chemistry where you will have the opportunity to feel like a real scientist, to carry out a series of chemical experiments, discover the history, immerse yourself into virtual reality and explore outer space. 1. Historical zone. Take a selfie in Mendeleev’s Cabinet This year, it is a century and a half since the creation of the Periodic Table. In 1869 there were no Internet, computers, smartphones and many other modern devices. We reconstructed the study of a chemist who worked in the nineteenth century. In the exhibition, visitors can see the basic scientific tools of that time, look at the first publication of the Table, and even take a selfie with its creator, Dmitrii Ivanovich Mendeleev. 2. Zone of space. Find out where it all came from How did hydrogen appear? And Iron? And what about Gold? Answers to these seemingly simple questions lie in the depths of the Universe.