Interactive Chemistry Exhibition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

The Quest to Explore the Heaviest Elements Raises Questions About How Far Researchers Can Extend Mendeleev’S Creation
ON THE EDGE OF THE PERIODIC TABLE The quest to explore the heaviest elements raises questions about how far researchers can extend Mendeleev’s creation. BY PHILIP BALL 552 | NATURE | VOL 565 | 31 JANUARY 2019 ©2019 Spri nger Nature Li mited. All ri ghts reserved. ©2019 Spri nger Nature Li mited. All ri ghts reserved. FEATURE NEWS f you wanted to create the world’s next undiscovered element, num- Berkeley or at the Joint Institute for Nuclear Research (JINR) in Dubna, ber 119 in the periodic table, here’s a possible recipe. Take a few Russia — the group that Oganessian leads — it took place in an atmos- milligrams of berkelium, a rare radioactive metal that can be made phere of cold-war competition. In the 1980s, Germany joined the race; I only in specialized nuclear reactors. Bombard the sample with a beam an institute in Darmstadt now named the Helmholtz Center for Heavy of titanium ions, accelerated to around one-tenth the speed of light. Ion Research (GSI) made all the elements between 107 and 112. Keep this up for about a year, and be patient. Very patient. For every The competitive edge of earlier years has waned, says Christoph 10 quintillion (1018) titanium ions that slam into the berkelium target Düllmann, who heads the GSI’s superheavy-elements department: — roughly a year’s worth of beam time — the experiment will probably now, researchers frequently talk to each other and carry out some produce only one atom of element 119. experiments collaboratively. The credit for creating later elements On that rare occasion, a titanium and a berkelium nucleus will collide up to 118 has gone variously, and sometimes jointly, to teams from and merge, the speed of their impact overcoming their electrical repul- sion to create something never before seen on Earth, maybe even in the Universe. -

Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants
UNLV Theses, Dissertations, Professional Papers, and Capstones 5-1-2015 Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants John Dustin Despotopulos University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Chemistry Commons Repository Citation Despotopulos, John Dustin, "Studies of Flerovium and Element 115 Homologs with Macrocyclic Extractants" (2015). UNLV Theses, Dissertations, Professional Papers, and Capstones. 2345. http://dx.doi.org/10.34917/7645877 This Dissertation is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Dissertation has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. INVESTIGATION OF FLEROVIUM AND ELEMENT 115 HOMOLOGS WITH MACROCYCLIC EXTRACTANTS By John Dustin Despotopulos Bachelor of Science in Chemistry University of Oregon 2010 A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy -

Python Module Index 79
mendeleev Documentation Release 0.9.0 Lukasz Mentel Sep 04, 2021 CONTENTS 1 Getting started 3 1.1 Overview.................................................3 1.2 Contributing...............................................3 1.3 Citing...................................................3 1.4 Related projects.............................................4 1.5 Funding..................................................4 2 Installation 5 3 Tutorials 7 3.1 Quick start................................................7 3.2 Bulk data access............................................. 14 3.3 Electronic configuration......................................... 21 3.4 Ions.................................................... 23 3.5 Visualizing custom periodic tables.................................... 25 3.6 Advanced visulization tutorial...................................... 27 3.7 Jupyter notebooks............................................ 30 4 Data 31 4.1 Elements................................................. 31 4.2 Isotopes.................................................. 35 5 Electronegativities 37 5.1 Allen................................................... 37 5.2 Allred and Rochow............................................ 38 5.3 Cottrell and Sutton............................................ 38 5.4 Ghosh................................................... 38 5.5 Gordy................................................... 39 5.6 Li and Xue................................................ 39 5.7 Martynov and Batsanov........................................ -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Synthesis of Superheavy Elements by Cold Fusion
Radiochim. Acta 99, 405–428 (2011) / DOI 10.1524/ract.2011.1854 © by Oldenbourg Wissenschaftsverlag, München Synthesis of superheavy elements by cold fusion By S. Hofmann1,2,∗ 1 GSI Helmholtzzentrum für Schwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany 2 Institut für Kernphysik, Goethe-Universität, Max von Laue-Straße 1, 60438 Frankfurt, Germany (Received February 3, 2011; accepted in revised form April 18, 2011) Superheavy elements / Synthesis / Cold fusion / in 1972, and the RILAC (variable-frequency linear acceler- Cross-sections / Decay properties / Recoil separators / ator) at the RIKEN Nishina Center in Saitama near Tokio in Detector systems 1980. In the middle of the 1960s the concept of the macrosco- pic-microscopic model for calculating binding energies Summary. The new elements from Z = 107 to 112 were of nuclei also at large deformations was invented by synthesized in cold fusion reactions based on targets of lead V. M. Strutinsky [3]. Using this method, a number of the and bismuth. The principle physical concepts are presented measured phenomena could be naturally explained by con- which led to the application of this reaction type in search sidering the change of binding energy as function of defor- experiments for new elements. Described are the technical mation. In particular, it became possible now to calculate the developments from early mechanical devices to experiments with recoil separators. An overview is given of present ex- binding energy of a heavy fissioning nucleus at each point of periments which use cold fusion for systematic studies and the fission path and thus to determine the fission barrier. synthesis of new isotopes. -

1. Purpose Evidence : Ready to Start with Paper, Pens Etc. out on Desk and Bags (With Phones in Them) Away Under the Seat
Per 3 Chemistry Class POWER Norms Evidence : 1. Purpose Ready to start with paper, pens etc. out on desk and bags (with phones in them) away under the seat. • • • • • • From Video: Periodic Table finished watching on Mon. Make notes to answer the following questions while watching: 1. Which elements are named the alkali metals? What is the trend in reactivity for the alkali metals? 2. What two factors led to the development of the current periodic table? 3. How are new elements made and named? 3. How are new elements made and named? Synthesis of New (heavy) elements: Decide on 2 elements that already exist that when combined will provide the desired number of protons Eg. 94Pu + 20Ca = 114Fl Step 1. Purify target material (eg.Plutonium) Step 2. Apply material to target. Step 3. Collide the second material (eg.Calcium) with target. Step 4. Separate newly formed heavy element atoms. 3. How are new elements made and named? Synthesis of New (heavy) elements: Decide on 2 elements that already exist thatThen when have combinedto quickly will provide the desired number of protons carry out prepared experiments to see if atoms have expected Eg. 94Pu + 20Ca = 114Fl properties compared to “homologues” Step 1. Purify target material (Plutonium) (atoms similar to it). Step 2. Apply material to target. Step 3. Collide the second material (Calcium) with target. Step 4. Separate newly formed heavy element atoms. How to name a New Element Elements are named after: A mythological concept of character, including an astronomical object; e.g. Neptunium, Plutonium A mineral or similar substance; e.g. -
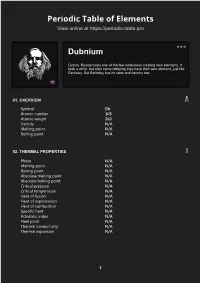
Dubnium Periodic Table of Elements
Periodic Table of Elements https://periodic-table.pro/Element/Db/enView online at https://periodic-table.pro Dubnium Dubna, Russia hosts one of the few institutions creating new elements. It took a while, but after some lobbying they have their own element, just like Berkeley. But Berkeley has its state and country too. 01. OVERVIEW Symbol Db Atomic number 105 Atomic weight 262 Density N/A Melting point N/A Boiling point N/A 02. THERMAL PROPERTIES Phase N/A Melting point N/A Boiling point N/A Absolute melting point N/A Absolute boiling point N/A Critical pressure N/A Critical temperature N/A Heat of fusion N/A Heat of vaporization N/A Heat of combustion N/A Specific heat N/A Adiabatic index N/A Neel point N/A Thermal conductivity N/A Thermal expansion N/A 03. PHYSICAL PROPERTIES Density N/A Density (liquid) N/A Molar volume N/A Molar mass 262 u Brinell hardness N/A Mohs hardness N/A Vickers hardness N/A Bulk modulus N/A Shear modulus N/A Young modulus N/A Poisson ratio N/A Refractive index N/A Speed of sound N/A Thermal conductivity N/A Thermal expansion N/A 04. REACTIVITY Valence 5 Electronegativity N/A Electron affinity N/A Ionization energies N/A 05. SAFETY Autoignition point N/A Flashpoint N/A Heat of combustion N/A 06. CLASSIFICATIONS Alternate names N/A Names of allotropes N/A Block, Group, Period d, 5, 7 Electron configuration [Rn] 5f¹⁴6d³7s² Color N/A Discovery 1967 in Russia and United States Gas phase N/A 07. -

Brief Encounters with Dubnium Lars Öhrström Tells of the Fleeting, but Still Tangible, Chemistry of Dubnium, the Heaviest of the Group 5 Elements
in your element Brief encounters with dubnium Lars Öhrström tells of the fleeting, but still tangible, chemistry of dubnium, the heaviest of the group 5 elements. ow you see me, now you don’t, this system for the separation of dubnium from might be a fitting exit line for a its group 4 neighbour rutherfordium4. Ndubnium atom slipping through our One has to be aware that a simple hands as it decays by sending out an alpha extrapolation from the properties of the particle. However, the chemist gets the last lighter group members (Nb and Ta in this laugh, as it is this very vanishing act that case) is not an adequate way of predicting the makes the one-atom-at-a-time chemistry properties of the 6d elements. As the nucleus experiments on element 105, a heavier gets heavier, relativistic effects come into play, analogue of tantalum, feasible. so the experiments need to be complemented As one of the most hotly disputed elements by advanced quantum chemistry. In this during the transfermium name dispute, case, such calculations suggest an increased when the Cold War cast a dark shadow on stability of the +5 oxidation state and an science, dubnium initially went under many electron configuration of [Rn]5f 146d37s2, names. It was synthesized by groups in the Legendary transuranium and transactinide concurrent with Db being the heaviest Soviet Union and the United States in the scientists Georgy Flerov (1913–1990), member of group 5 (ref. 1). late 1960s, starting in Dubna — the science Yuri Oganessian (1933–) and Glenn Seaborg This intricate interplay between theory city outside Moscow that was to eventually (1912–1999) on the Volga River bank in Dubna, and experiment is of interest and importance give its name to the element. -

What's Genius ? Dmitrii Mendeleev
WHY GENIUS ? WHAT’S GENIUS ? DMITRII MENDELEEV: THE TABLE AND BEYOND VLADIMIR SHILTSEV FERMI NATIONAL ACCELERATOR LABORATORY, BATAVIA, IL, USA EUROPEAN PHYSICAL SOCIETY AMERICAN PHYSICAL SOCIETY HTTPS://WWW.IYPT2019.ORG/ UNESCO INT’L YEAR OF PERIODIC TABLE IYPT’2019 MAP Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 2 Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 3 FERMILAB (CHICAGO) – MAY 2019 Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 4 Prof.Vladimir Petra Shiltsev Rudolf | Mendeleev(Netherlands - CERN, Nov )2019 5 Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 6 DMITRII IVANOVICH MENDELEEV (DIM) GENIUS “THE TABLE” AND BEYOND VLADIMIR SHILTSEV CERN 2019 Maxwell Darwin Pavlov GaribaldiVladimir Shiltsev | Mendeleev - CERN, Nov 2019Tolstoy Marx 8 DIM : b. 1834 (Tobolsk), d. 1907 (StPetersburg) 1869 1850 1900 Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 9 DIM’S ORIGINAL TABLE FEB. 17, 1869 Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 10 IMPORTANT SUBTLETY: MAJOR DISCOVERY IS PERIODIC LAW, THE TABLE IS JUST ITS PRESENTATION • PERIODIC LAW: PERIODIC DEPENDENCE OF CHEMICAL PROPERTIES OF ELEMENTS ON THEIR ATOMIC WEIGHT • PRESENTED: 6 MARCH 1869 • INITIALLY: ONLY 63 ELEMENTS • PREDICTED AND DISCOVERED: GA (1875), SC (1879), GE (1885) Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 11 DISCOVERY OF GALLIUM 1875 - Paul-Émile Lecoq de Boisbaudran – original density measured 4.7 g/cm3, Mendeleev advised to re-measure – the correct value found was 5.9 g/cm3 1871 1875 ability to act either as an acid or a base. Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 12 WHY (THE TABLE)? • DISCOVERY OF ELECTRON (1897), PROTON (1913) AND NEUTRON (1932) • CHEMICAL PROPERTIES EXPLAINED BY QUANTUM MECHANICS • COMPOSITION OF NUCLEI – BY QUANTUM CHROMODYNAMICS 13 Vladimir Shiltsev | Mendeleev - CERN, Nov 2019 29 Man-Made Synthetic Elements Element Symbol At.