Synthesis of Superheavy Elements by Cold Fusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

NOBELIUM Element Symbol: No Atomic Number: 102
NOBELIUM Element Symbol: No Atomic Number: 102 An initiative of IYC 2011 brought to you by the RACI KERRY LAMB www.raci.org.au NOBELIUM Element symbol: No Atomic number: 102 The credit for discovering Nobelium was disputed with 3 different research teams claiming the discovery. While the first claim dates back to 1957, it was not until 1992 that the International Union of Pure and Applied Chemistry credited the discovery to a research team from Dubna in Russia for work they did in 1966. The element was named Nobelium in 1957 by the first of its claimed discoverers (the Nobel Institute in Sweden). It was named after Alfred Nobel, a Swedish chemist who invented dynamite, held more than 350 patents and bequeathed his fortune to the establishment of the Nobel Prizes. Nobelium is a synthetic element and does not occur in nature and has no known uses other than in scientific research as only tiny amounts of the element have ever been produced. Nobelium is radioactive and most likely metallic. The appearance and properties of Nobelium are unknown as insufficient amounts of the element have been produced. Nobelium is made by the bombardment of curium (Cm) with carbon nuclei. Its most stable isotope, 259No, has a half-life of 58 minutes and decays to Fermium (255Fm) through alpha decay or to Mendelevium (259Md) through electron capture. Provided by the element sponsor Freehills Patent and Trade Mark Attorneys ARTISTS DESCRIPTION I wanted to depict Alfred Nobel, the namesake of Nobelium, as a resolute young man, wearing the Laurel wreath which is the symbol of victory. -
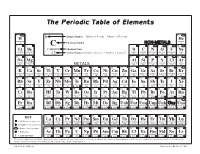
The Periodic Table of Elements
The Periodic Table of Elements 1 2 Atomic Number = Number of Protons = Number of Electrons H 6 He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo FRANCIUM RADIUM -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

An Octad for Darmstadtium and Excitement for Copernicium
SYNOPSIS An Octad for Darmstadtium and Excitement for Copernicium The discovery that copernicium can decay into a new isotope of darmstadtium and the observation of a previously unseen excited state of copernicium provide clues to the location of the “island of stability.” By Katherine Wright holy grail of nuclear physics is to understand the stability uncover its position. of the periodic table’s heaviest elements. The problem Ais, these elements only exist in the lab and are hard to The team made their discoveries while studying the decay of make. In an experiment at the GSI Helmholtz Center for Heavy isotopes of flerovium, which they created by hitting a plutonium Ion Research in Germany, researchers have now observed a target with calcium ions. In their experiments, flerovium-288 previously unseen isotope of the heavy element darmstadtium (Z = 114, N = 174) decayed first into copernicium-284 and measured the decay of an excited state of an isotope of (Z = 112, N = 172) and then into darmstadtium-280 (Z = 110, another heavy element, copernicium [1]. The results could N = 170), a previously unseen isotope. They also measured an provide “anchor points” for theories that predict the stability of excited state of copernicium-282, another isotope of these heavy elements, says Anton Såmark-Roth, of Lund copernicium. Copernicium-282 is interesting because it University in Sweden, who helped conduct the experiments. contains an even number of protons and neutrons, and researchers had not previously measured an excited state of a A nuclide’s stability depends on how many protons (Z) and superheavy even-even nucleus, Såmark-Roth says. -

Vol. 25 No. 3 (2015)
Nuclear Physics News International Volume 25, Issue 3 July–September 2015 FEATURING: RIKEN • Neutron-Antineutron Oscillations at ESS • HIgS • SUBARU 10619127(2015)25(3) Physics Magazines Editor: Editor: Editors: Hannu Mutka, ILL Gabriele-‐Elisabeth Körner, NuPECC Ronald Frahm, Univ. of Wuppertal Michael C. Martin, ALS Motohiro Suzuki, SPring-‐8 You can learn more about the physics magazines published by Taylor & Francis, as well view as current and past editorial topics and sign up for : alerts at www.tandfonline.com Please contact Maureen Williams [email protected] ( ) for advertising opportunities. Nuclear Physics News Volume 25/No. 3 Nuclear Physics News is published on behalf of the Nuclear Physics European Collaboration Committee (NuPECC), an Expert Committee of the European Science Foundation, with colleagues from Europe, America, and Asia. Editor: Gabriele-Elisabeth Körner Editorial Board Maria José Garcia Borge, Madrid (Chair) Eugenio Nappi, Bari Rick Casten, Yale Klaus Peters, Darmstadt and EPS/NPB Jens Dilling, Vancouver Hermann Rothard, Caen Ari Jokinen, Jyväskylä Hideyuki Sakai, Tokyo Yu-Gang Ma, Shanghai James Symons, Berkeley Douglas MacGregor, Glasgow and EPS/NPB Editorial Office:Physikdepartment, E12, Technische Universitat München, 85748 Garching, Germany, Tel: +49 89 2891 2293, +49 172 89 15011, Fax: +49 89 2891 2298, E-mail: [email protected] Correspondents (from countries not covered by the Editorial Board and NuPECC) Argentina: O. Civitaresse, La Plata; Australia: A. W. Thomas, Adelaide; Brasil: M. Hussein, São Paulo; India: D. K. Avasthi, New Delhi; Israel: N. Auerbach, Tel Aviv; Mexico: E. Padilla-Rodal, Mexico DF; Russia: Yu. Novikov, St. Petersburg; Serbia: S. Jokic, Belgrade; South Africa: S. -

Darmstadtium, Roentgenium and Copernicium Form Strong Bonds with Cyanide
Darmstadtium, Roentgenium and Copernicium Form Strong Bonds With Cyanide Taye B. Demissie∗and Kenneth Ruudy March 23, 2017 Abstract We report the structures and properties of the cyanide complexes of three super- heavy elements (darmstadtium, roentgenium and copernicium) studied using two- and four-component relativistic methodologies. The electronic and structural properties of these complexes are compared to the corresponding complexes of platinum, gold and mercury. The results indicate that these superheavy elements form strong bonds with cyanide. Moreover, the calculated absorption spectra of these superheavy-element cyanides show similar trends to those of the corresponding heavy-atom cyanides. The calculated vibrational frequencies of the heavy-metal cyanides are in good agreement with available experimental results lending support to the quality of our calculated vibrational frequencies for the superheavy-atom cyanides. ∗Centre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic Uni- versity of Norway, N-9037 Tromsø, Norway yCentre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic Uni- versity of Norway, N-9037 Tromsø, Norway 1 Relativistic two- and four-component density-functional theory is used to demonstrate that the superheavy elements darmstadtium, roentgenium and copernicium form stable complexes with cyanide, providing new insight into the chemistry of these superheavy elements. 2 INTRODUCTION The term heavy atom refers roughly to elements in the 4th - -

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -

Cold Fusion Again 1 Introduction
Prespacetime Journal j February 2016 j Volume 7 j Issue 2 j pp. 379-396 379 Pitk¨anen,M., Cold Fusion Again Exploration Cold Fusion Again Matti Pitk¨anen 1 Abstract During years I have developed two models of cold fusion and in this article these models are combined together. The basic idea of TGD based model of cold is that cold fusion occurs in two steps. First dark nuclei (large heff = n × h) with much lower binding energy than ordinary nuclei are formed at magnetic flux tubes possibly carrying monopole flux. These nuclei can leak out the system along magnetic flux tubes. Under some circumstances these dark nuclei can transform to ordinary nuclei and give rise to detectable fusion products. An essential additional condition is that the dark protons can decay to neutrons rapidly enough by exchanges of dark weak bosons effectively massless below atomic length scale. Also beta decays in which dark W boson decays to dark electron and neutrino can be considered. This allows to overcome the Coulomb wall and explains why final state nuclei are stable and the decay to ordinary nuclei does not yield only protons. Thus it seems that this model combined with the TGD variant of Widom-Larsen model could explain nicely the existing data. In this chapter I will describe the steps leading to the TGD inspired model for cold fusion combining the earlier TGD variant of Widom-Larsen modelwith the model inspired by the TGD inspired model of Pollack's fourth phase of water using as input data findings from laser pulse induced cold fusion discovered by Leif Holmlid and collaborators. -

Cold Nuclear Fusion Reactor and Nuclear Fusion Rocket
American Journal of Engineering Research (AJER) 2013 American Journal of Engineering Research (AJER) e-ISSN : 2320-0847 p-ISSN : 2320-0936 Volume-02, Issue-10, pp-401-410 www.ajer.org Research Paper Open Access Cold nuclear fusion reactor and nuclear fusion rocket Huang Zhenqiang and Huang Yuxiang Administration of Fujian Chemical Geology and Mines Geological Exploration Institute of Chemical Jinan District, Fuzhou, Fujian, China West Yuen Road 68 West Phoenix Abstract: - "Nuclear restraint inertial guidance directly hit the cold nuclear fusion reactor and ion speed dc transformer" [1], referred to as "cold fusion reactor" invention patents, Chinese Patent Application No. CN: 200910129632.7 [2]. The invention is characterized in that: at room temperature under vacuum conditions, specific combinations of the installation space of the electromagnetic field, based on light nuclei intrinsic magnetic moment and the electric field, the first two strings of the nuclei to be bound fusion on the same line (track) of. Re-use nuclear spin angular momentum vector inherent nearly the speed of light to form a super strong spin rotation gyro inertial guidance features, to overcome the Coulomb repulsion strong bias barrier to achieve fusion directly hit. Similar constraints apply nuclear inertial guidance mode for different speeds and energy ion beam mixing speed, the design of ion speed dc transformer is cold fusion reactors, nuclear fusion engines and such nuclear power plants and power delivery systems start important supporting equipment, so apply for a patent merger. Keywords: - Cold Fusion Reactor, Nuclear restraint, Inertial guidance, direct fusion of the collision, nuclear magnetic moment, Spin angular momentum vector, nearly the speed of light spin, Rotation of the gyro inertial guidance features, Ion speed dc transformer. -

Gas-Phase Chemistry of Element 114, Flerovium
EPJ Web of Conferences 131, 07003 (2016) DOI: 10.1051/epjconf/201613107003 Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements Gas-phase chemistry of element 114, flerovium Alexander Yakushev1,2,a and Robert Eichler3,4 1 GSI Helmholtzzentrum für Schwerionenforschung GmbH, 64291 Darmstadt, Germany 2 Helmholtz-Institut Mainz, 55099 Mainz, Germany 3 Paul Scherrer Institut, 5232 Villigen PSI, Switzerland 4 University of Bern, Department of Chemistry and Biochemistry, 3012 Bern, Switzerland Abstract. Element 114 was discovered in 2000 by the Dubna-Livermore collaboration, and in 2012 it was named flerovium. It belongs to the group 14 of the periodic table of elements. A strong relativistic stabilisation of 2 2 the valence shell 7s 7p1/2 is expected due to the orbital splitting and the 2 2 contraction not only of the 7s but also of the spherical 7p1/2 closed subshell, resulting in the enhanced volatility and inertness. Flerovium was studied chemically by gas-solid chromatography upon its adsorption on a gold surface. Two experimental results on Fl chemistry have been published so far. Based on observation of three atoms, a weak interaction of flerovium with gold was suggested in the first study. Authors of the second study concluded on the metallic character after the observation of two Fl atoms deposited on gold at room temperature. 1. Introduction Man-made superheavy elements (SHE, atomic number Z ≥ 104) are unique in two aspects. Their nuclei exist only due to nuclear shell effects, and their electron structure is influenced by increasingly important relativistic effects [1–3]. Recently the synthesis of elements 113, 115, 117 and 118 has been approved, thus, the discovery of all elements of the 7th row in the periodic table of elements with proton number Z up to 118 has been acknowledged [4].