Uncovering the Periodic Table of Elements
Total Page:16
File Type:pdf, Size:1020Kb
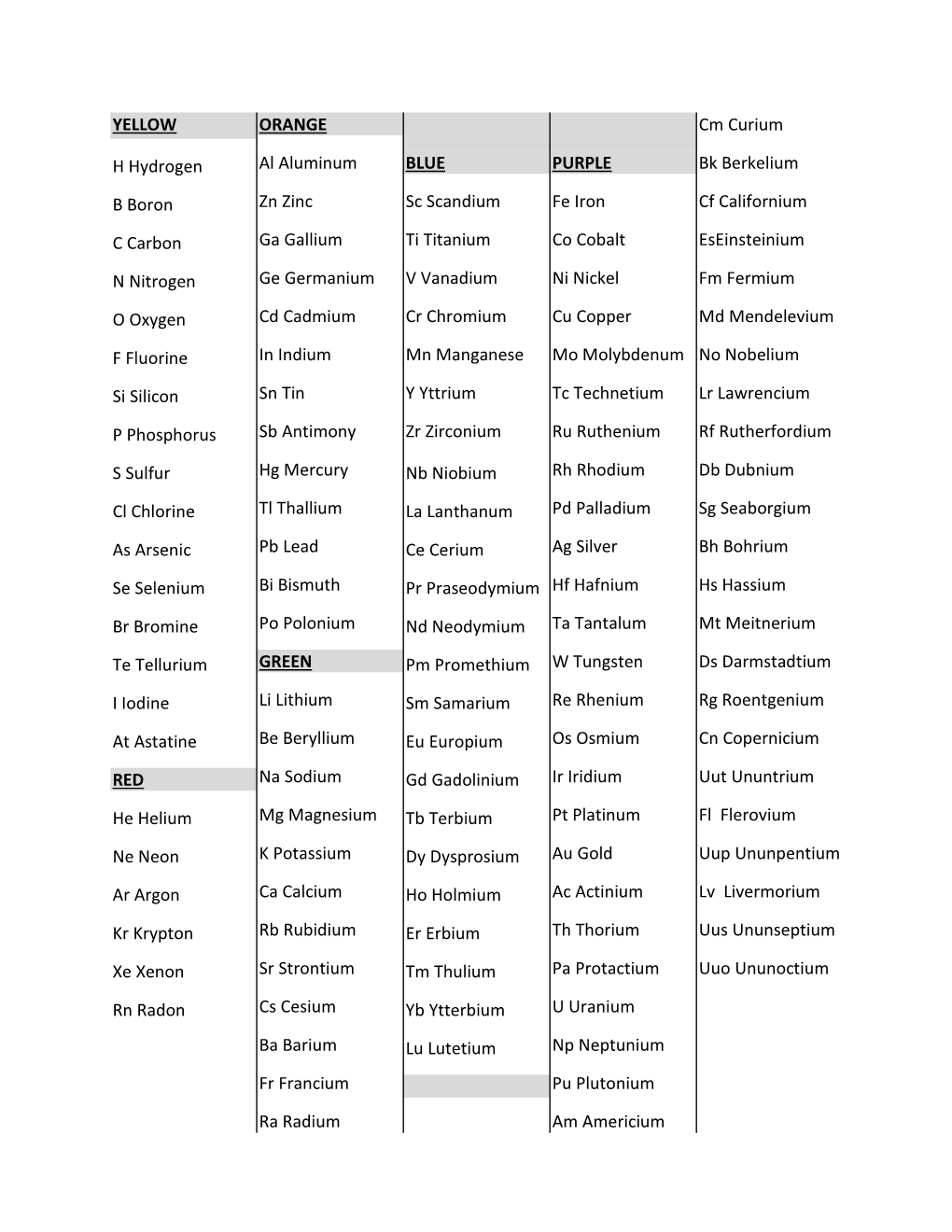
Load more
Recommended publications
-

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -
The Periodic Table of Elements
The Periodic Table of Elements 1 2 Atomic Number = Number of Protons = Number of Electrons H 6 He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo FRANCIUM RADIUM -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

FRANCIUM Element Symbol: Fr Atomic Number: 87
FRANCIUM Element Symbol: Fr Atomic Number: 87 An initiative of IYC 2011 brought to you by the RACI KAYE GREEN www.raci.org.au FRANCIUM Element symbol: Fr Atomic number: 87 Francium (previously known as eka-cesium and actinium K) is a radioactive metal and the second rarest naturally occurring element after Astatine. It is the least stable of the first 103 elements. Very little is known of the physical and chemical properties of Francium compared to other elements. Francium was discovered by Marguerite Perey of the Curie Institute in Paris, France in 1939. However, the existence of an element of atomic number 87 was predicted in the 1870s by Dmitri Mendeleev, creator of the first version of the periodic table, who presumed it would have chemical and physical properties similar to Cesium. Several research teams attempted to isolate this missing element, and there were at least four false claims of discovery during which it was named Russium (after the home country of soviet chemist D. K. Dobroserdov), Alkalinium (by English chemists Gerald J. K. Druce and Frederick H. Loring as the heaviest alkali metal), Virginium (after Virginia, home state of chemist Fred Allison), and Moldavium (by Horia Hulubei and Yvette Cauchois after Moldavia, the Romanian province where they conducted their work). Perey finally discovered Francium after purifying radioactive Actinium-227 from Lanthanum, and detecting particles decaying at low energy levels not previously identified. The new product exhibited chemical properties of an alkali metal (such as co-precipitating with Cesium salts), which led Perey to believe that it was element 87, caused by the alpha radioactive decay of Actinium-227. -

Microdosimetry of Astatine-211 and Xa0102708 Comparison with That of Iodine-125
MICRODOSIMETRY OF ASTATINE-211 AND XA0102708 COMPARISON WITH THAT OF IODINE-125 T. UNAK Ege University, Bomova, Izmir, Turkey Abstract.' 21lAt is an alpha and Auger emitter radionuclide and has been frequently used for labeling of different kind of chemical agents. 125I is also known as an effective Auger emitter. The radionuclides which emit short range and high LET radiations such as alpha particles and Auger electrons have high radiotoxic effectiveness on the living systems. The microdosimetric data are suitable to clarify the real radiotoxic effectiveness and to get the detail of diagnostic and therapeutic application principles of these radionuclides. In this study, the energy and dose absorptions by cell nucleus from alpha particles and Auger electrons emitted by 211At have been calculated using a Monte Carlo calculation program (code: UNMOC). For these calculations two different model corresponding to the cell nucleus have been used and the data obtained were compared with the data earlier obtained for I25I. As a result, the radiotoxicity of 21lAt is in the competition with 125I. In the case of a specific agent labelled with 2I1At or 125I is incorporated into the cell or cell nucleus, but non-bound to DNA or not found very close to it, 2llAt should considerably be much more radiotoxic than i25I, but in the case of the labelled agent is bound to DNA or take a place very close to it, the radiotoxicity of i25I should considerably be higher than 21'At. 1. INTRODUCTION 21'At as an alpha and Auger emitter radionuclide has an extremely high potential application in cancer therapy; particularly, in molecular radiotherapy. -

Darmstadtium, Roentgenium and Copernicium Form Strong Bonds with Cyanide
Darmstadtium, Roentgenium and Copernicium Form Strong Bonds With Cyanide Taye B. Demissie∗and Kenneth Ruudy March 23, 2017 Abstract We report the structures and properties of the cyanide complexes of three super- heavy elements (darmstadtium, roentgenium and copernicium) studied using two- and four-component relativistic methodologies. The electronic and structural properties of these complexes are compared to the corresponding complexes of platinum, gold and mercury. The results indicate that these superheavy elements form strong bonds with cyanide. Moreover, the calculated absorption spectra of these superheavy-element cyanides show similar trends to those of the corresponding heavy-atom cyanides. The calculated vibrational frequencies of the heavy-metal cyanides are in good agreement with available experimental results lending support to the quality of our calculated vibrational frequencies for the superheavy-atom cyanides. ∗Centre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic Uni- versity of Norway, N-9037 Tromsø, Norway yCentre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic Uni- versity of Norway, N-9037 Tromsø, Norway 1 Relativistic two- and four-component density-functional theory is used to demonstrate that the superheavy elements darmstadtium, roentgenium and copernicium form stable complexes with cyanide, providing new insight into the chemistry of these superheavy elements. 2 INTRODUCTION The term heavy atom refers roughly to elements in the 4th - -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

Enigmatic Astatine D
in your element Enigmatic astatine D. Scott Wilbur points out the difficulty in studying the transient element astatine, and the need to understand its basic chemical nature to help in the development of targeted radiotherapy agents. ince the discovery of astatine The other ‘long’-lived isotope, 210At, is not of labelling reagents containing more over 70 years ago1, many of its suitable because it decays into polonium-210 stable aromatic astatine–boron bonds Scharacteristics have remained elusive. — the notorious radiation poison used to has improved that situation, and studies Unlike the other halogens, abundant and kill the Russian Federal Security Service evaluating bonding with other elements ubiquitous in nature, astatine is one of officer Alexander Litvinenko in 2006, may further advance it. the rarest of all elements. This arises from after he took political asylum in the To determine in vivo stability, the the fact that it has no stable isotopes; the United Kingdom. same cancer-targeting molecule can longest lived of its 32 known radioisotopes, Although some chemical data has be labelled with 211At and (stably) with 210At, has a half-life of only 8.1 hours. been compiled for astatine isotopes, radioiodine (125I, 123I or 131I), and the two The rarity and radioactive nature of many physical properties have only been co-injected. The concentrations of 211At element 85 lends to its mystery, as it cannot extrapolated. Similar to other halogens, in various tissues (higher lung, spleen, be observed or weighed in a conventional astatine undergoes nucleophilic and stomach and thyroid) indicate whether it is sense. Even its colour is unknown; based electrophilic reactions. -

ROENTGENIUM Element Symbol: Rg Atomic Number: 111
ROENTGENIUM Element Symbol: Rg Atomic Number: 111 An initiative of IYC 2011 brought to you by the RACI PATRICIA ZUBER www.raci.org.au ROENTGENIUM Element symbol: Rg Atomic number: 111 Roentgenium has the symbol Rg and atomic number 111. However, it is not a stable element and can only be produced by cold fusion between other elements (for example by bombarding bismuth with nickel). Once formed, it is a synthetic radioactive element that falls apart in less than 20 sec. Therefore, there is currently no direct use for it. This element was first discovered by the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany in 1994. GSI also proposed a name for this new element: Roentgenium. They honoured the achievements of Wilhelm Conrad Röntgen, a physicist, who discovered X-rays in 1895 while being professor at the University of Wuerzburg in Germany. Röntgen is considered the father of diagnostic radiology, the medical specialty which uses imaging to diagnose disease. Roentgen never patented anything because he believed that knowledge should be available for everybody. He received the very first Nobel Prize in Physics in 1901. Provided by the element sponsor Martina Stenzel ARTISTS DESCRIPTION Lino cut, silk screen Roentgenium is a synthetic radioactive element produced by cold fusion. It has a half life of 20 seconds and decays by spontaneous fission. Not an easy element to create an image from! I decided to use a diagram of its atomic structure and fission lines as a basis for a relief image. I cut a paper stencil of Rg 111 and silk screened it in silver across the centre of this printed image. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

Three Related Topics on the Periodic Tables of Elements
Three related topics on the periodic tables of elements Yoshiteru Maeno*, Kouichi Hagino, and Takehiko Ishiguro Department of physics, Kyoto University, Kyoto 606-8502, Japan * [email protected] (The Foundations of Chemistry: received 30 May 2020; accepted 31 July 2020) Abstaract: A large variety of periodic tables of the chemical elements have been proposed. It was Mendeleev who proposed a periodic table based on the extensive periodic law and predicted a number of unknown elements at that time. The periodic table currently used worldwide is of a long form pioneered by Werner in 1905. As the first topic, we describe the work of Pfeiffer (1920), who refined Werner’s work and rearranged the rare-earth elements in a separate table below the main table for convenience. Today’s widely used periodic table essentially inherits Pfeiffer’s arrangements. Although long-form tables more precisely represent electron orbitals around a nucleus, they lose some of the features of Mendeleev’s short-form table to express similarities of chemical properties of elements when forming compounds. As the second topic, we compare various three-dimensional helical periodic tables that resolve some of the shortcomings of the long-form periodic tables in this respect. In particular, we explain how the 3D periodic table “Elementouch” (Maeno 2001), which combines the s- and p-blocks into one tube, can recover features of Mendeleev’s periodic law. Finally we introduce a topic on the recently proposed nuclear periodic table based on the proton magic numbers (Hagino and Maeno 2020). Here, the nuclear shell structure leads to a new arrangement of the elements with the proton magic-number nuclei treated like noble-gas atoms. -

Unit 6 the Periodic Table How to Group Elements Together? Elements of Similar Properties Would Be Group Together for Convenience
Unit 6 The periodic table How to group elements together? Elements of similar properties would be group together for convenience. The periodic table Chemists group elements with similar chemical properties together. This gives rise to the periodic table. In the periodic table, elements are arranged according to the following criteria: 1. in increasing order of atomic numbers and 2. according to the electronic arrangement The diagram below shows a simplified periodic table with the first 36 elements listed. Groups The vertical columns in the periodic table are called groups . Groups are numbered from I to VII, followed by Group 0 (formerly called Group VIII). [Some groups are without group numbers.] The table below shows the electronic arrangements of some elements in some groups. Group I Group II Group VII Group 0 He (2) Li (2,1) Be (2,2) F (2,7) Ne (2,8) Na (2,8,1) Mg (2,8,2) Cl (2,8,7) Ar (2,8,8) K (2,8,8,1) Ca (2,8,8,2) Br (2,8,18,7) Kr (2,8,18,8) What is the relationship between the group numbers and the electronic arrangements of the elements? Group number = the number of outermost shell electrons in an atom of the element The chemical properties of an element depend mainly on the number of outermost shell electrons in its atoms. Therefore, elements within the same group would have similar chemical properties and would react in a similar way. However, there would be a gradual change of reactivity of the elements as we move down the group.