Biodetection Technologies for First Responders: 2014 Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LAB: One Tube Reaction Part 1
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube, iron nail, sodium chloride, copper (II) sulfate, distilled water, glass stirring rod, tissue paper, parafilm wax Procedures: 1. Label a test tube to identify your group 2. Fill approx. 1/3 of the test tube with copper (II) sulfate crystals. Gently tap the tube to allow the crystals to settle. 3. Using a glass stirring rod, carefully cover the crystals with a layer of Kimwipe tissue paper. 4. Slowly, and with as little disturbance as possible, add enough distilled water to just cover the paper and blue crystals. 5. Repeat the process for the sodium chloride, filling approx. 1/3 of the test tube. Gently tap the tube to allow the crystals to settle. 6. Push more tissue paper into the test tube on top of the white crystals. 7. Add enough water to cover the tissue paper and white crystals. 8. Obtain an iron nail and expose the surface by rubbing with sand paper. 9. Carefully slide the nail into the test tube. 10. Continue adding water until the nail is completely covered. 11. Cover the test tube with parafilm wax and record your Day 1 observations. 12. Return the test tube to the test tube rack. 13. Continue to monitor and record your observations for the next several days, as indicated in the data table. Data: 1. Complete the NFPA label for the two chemicals used in this lab. -

The Science of the Bioeconomy
The science of the Bioeconomy Dr. Henrike Gebhardt 05 December 2014 Our positioning Evonik is the creative industrial group from Germany and one of the world’s leading specialty chemicals companies. The Science of the Bioeconomy Page 3 Our credo The Bioeconomy is one driver to promote a more resource-efficient and sustainable economy. Industrial biotechnology is a key technology for realising the bioeconomy. The Science of the Bioeconomy Page 5 Overview Bioeconomy Biotechnology Genetic engineering The Science of the Bioeconomy Page 6 Definitions Bioeconomy Production of renewable biological resources and the conversion of these resources and waste streams into value added products, such as food, feed, and other industrial products and energy. COM(2012) 60, EU Commission, mod. Bio-basedBiotechnology products ProductsThe use whollyof living or organisms partly derived or their from components biomass. EN to16575 make products. Genetic engineering Any of various applications of biological science used in the manipulation of the genome of an organism The Science of the Bioeconomy Page 7 Bio-based products offered by Evonik Polyamids Polyesters VESTAMID ®Terra DYNACOLL ®Terra DYNAPOL ®Terra VISIOMER ®Terra Additives Amino acids Cosmetics BioMTBE Feed additives Health – purified TEGOSOFT ®MM bio-based AdditivesCleaning Health VISCOPLEX ® Series 10 Esterquats RESOMER ® bio- degradable The Science of the Bioeconomy Page 8 Evonik invests in high-growth chemical megatrends Lighthouse investment projects Lysine Russia Consumer Specialties China C4 Chemistry H O / HPPO Europe 2 2 Lysine Expansion China USA Crosslinkers, Isophorone China Consumer Specialties Superabsorbents Brazil Saudi Arabia Methionine Singapore Biodiesel catalysts Argentina Bioeconomy Lysine Traditional Brazil The Science of the Bioeconomy Page 9 Bioeconomy Press releases Company Raw Intermediate Product Material Date of Issue Volume Commissioning DSM/POET (USA) Cellulosics Ethanol Biofuels from corn Jan 2012 90 kta H1.2014 cobs Purac/BASF (ES) Cellulosics Succinic acid e. -

Equipment Detailsr07
Lab Equipment Details Lab Equipment Glass Flasks 150ml 250ml 500ml Lab Equipment Glass Beakers 150ml 250ml 500ml Lab Equipment Glassware But once removed, only the cap stays highlighted. Droppers critical to Lab An activated Dropper coursework can be found highlights the entire bottle. already in the workspace. Dropper Dropper Dropper Activated In Use Lab Equipment Gastight Syringe Small A pre-filled gastight syringe can be used with a NMR tube to safely fill through the top in preparation for use with the NMR spectrometer. Gastight Syringe NMR Tube with Holder with Holder Lab Equipment NMR Tube Spinner An NMR Tube filled with gas for use with the NMR Spectrometer Simply use the NMR Tube with needs to be inserted into the the Tube Spinner. Spinner before it can be used. NMR Tube NMR Tube Spinner NMR Tube Spinner with Holder with NMR Tube inserted Once the Tube slotted into the holder is inserted into the top part of the spectrometer, users can type in a number for Lab Equipment the frequency they’d like to scan. NMR Spectrometer XL The NMR Tube holder can then be slotted into the highlighted tube on the NMR Spectrometer. NMR Tube filled with appropriate substance is slotted (used with) in the holder. Lift will perpare the Spectrometer for the tube holder insertion. Scan No. Allows the user to change the frequency at which the tube is scanned The NMR Tube holder can then be slotted into the top tube on Lab Equipment the NMR Spectrometer. NMR Spectrometer XL NMR Tube filled with appropriate substance is slotted (used with) in the holder. -

International Handbook of Foodborne Pathogens
INTERNATIONAL HANDBOOK OF FOODBORNE PATHOGENS EDITED BY MARIANNE D. MILIOTIS U.S. Food and Drug Administration College Park, Maryland, U.S.A. JEFFREY W. BIER Food Safety Consultant Alexandria, Virginia, U.S.A. MARCEL H MARCEL DEKKER, INC. NEW YORK • BASEL Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved. Library of Congress Cataloging-in-Publication Data A catalog record for this book is available from the Library of Congress. ISBN: 0-8247-0685-4 This book is printed on acid-free paper. Headquarters Marcel Dekker, Inc. 270 Madison Avenue, New York, NY 10016 tel: 212-696-9000; fax: 212-685-4540 Eastern Hemisphere Distribution Marcel Dekker AG Hutgasse 4, Postfach 812, CH-4001 Basel, Switzerland tel: 41-61-260-6300; fax: 41-61-260-6333 World Wide Web http://www.dekker.com The publisher offers discounts on this book when ordered in bulk quantities. For more information, write to Special Sales/Professional Marketing at the headquarters address above. Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage and retrieval system, without permission in writing from the publisher. Current printing (last digit): 10987654321 PRINTED IN THE UNITED STATES OF AMERICA Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved. FOOD SCIENCE AND TECHNOLOGY A Series of Monographs, Textbooks, and Reference Books EDITORIAL BOARD Senior Editors Owen R. Fennema University of Wisconsin-Madison Y. H. Hui Science Technology System Marcus Karel Rutgers University (emeritus) Pieter Walstra Wagenmgen University John R. -

Documm Mgson!
Documm mgson! ED 174 412 SE 027 981 TITLE Biomedical Science, Unit I: Respiration inHealth and Medicine. Respiratory Anatomy, Physiology and Pathology; The Behavior of Gases;Introductory Chemistry; and Air Pollution. Laboratory Manual. Revised Version, 1975. INSTITUTION Biomedical Interdisciplinary Curriculum Project, Berkeley, Calif. SPONS AGENCY National Science Foundation, Washington, D.C. PUB DATE 75 NOTE 181p.; For related documents, see SE 027978-999 and SE 028 510-516; Not available in hard copydue to copyright restrictions EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIpTCRS Chemistry; Environment; Health; *HealthEducation; Higher Education; *Laboratory ExFeriments; *Laboratory Techniques; *Laboratory Training;Medical Education; Pathology; Physics; *Physiology;*Science Education; Secondary Education IDENTIFIERS *Respiration ABSTItACT Designed to accompany the student text on respiration, this manual presents instructions on the use of labcra tcry equipment and presents various experimentsdealing with the concepts presented in the text. Thirty=nine laboratoryactivities ' are de scribed. Laboratory activities are dividedinto several parts, each Part covering a specific experiment dealing with the concept covered by the activity. Each experimentincludes a description of materials, procedures, and discurision questions. (RE) ********************************************************************** Reproductions supplied by EDRS are the bestthat can be made from the original document. 4********************M414141#***********4141*****4141***********41#********4 -
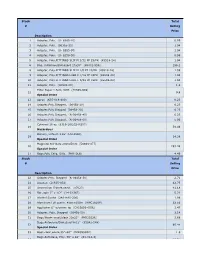
Stock Total # Selling Price 1 Adapter, Poly. (U- 6365-10)
Stock Total # Selling Price Description 1 Adapter, Poly. (U- 6365-10) 0.99 2 Adapter, Poly. (06365-22) 1.04 3 Adapter, Poly. (U- 6365-30) 1.04 4 Adapter, Poly. (U- 6359-50) 0.99 5 Adapter, Poly,FITTINGS SLIP M 3/32 PP 25/PK (45518-24) 1.04 6 Bag, Autoclave/Biohazard 24x30'' (95042-556) 156.1 8 Adapter, Poly.FITTINGS SLIP M 1/8 PP 25/PK (45518-26) 1.59 9 Adapter, Poly.FITTINGS LUER F 1/16 PP 25PK (45508-00) 1.04 10 Adapter, Poly.FITTINGS LUER F 3/32 PP 25PK (45508-02) 1.04 11 Adapter, Poly. (30800-08) 1.3 Filter Paper 1.5cm, VWR (28309-989) 12 9.9 Special Order 13 Apron (635-018-400) 0.23 14 Adapter,Poly.Stepped, (06458-10) 6.23 15 Adapter,Poly.Stepped (06458-20) 6.23 16 Adapter,Poly.Stepped, (N-06458-40) 6.23 17 Adapter,Poly.Stepped, (N-06458-60) 5.06 Cytoseal 16 oz (8310-16/23244257) 18 58.49 Hazardous Battery, Lithium 3.6V (LS14500) 19 14.38 Special Order Magnetic Stir Bars 2mmx5mm (58948-377) 20 293.48 Special Order 21 Bags,Poly Cello,10 lb. (PKR10Lb) 4.49 Stock Total # Selling Price Description 22 Adapter,Poly. Stepped (N-06458-30) 2.71 24 Alconox (21835-032) 42.75 25 Ammonium Sulfate,certif. (A7023) 41.18 26 Bar, spin 2'' x 1/2'' (14-51367) 5.22 27 Alcohol Swabs (248-HAS-200) 1.96 28 Aluminum Foil comm. 45cmx100m (WPC1810P) 32.56 29 Applicator 6'' w/cotton tip (CA10806-000L) 1.47 30 Adapter, Poly. -

Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017
EPA/600/R-17/356 | September 2017 www.epa.gov/homeland-security-research Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017 Office of Research and Development Homeland Security Research Program This page left intentionally blank EPA/600/R-17/356 | September 2017 Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY Cincinnati, OH 45268 Office of Research and Development Homeland Security Research Program Disclaimer Disclaimer The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development funded and managed the research described here under Contract EP-C-15-012 to CSRA Inc. This document is undergoing review and has not been approved for publication. The contents reflect the views of the contributors and technical work groups and do not necessarily reflect the views of the Agency. Mention of trade names or commercial products in this document or in the methods referenced in this document does not constitute endorsement or recommendation for use. Questions concerning this document or its application should be addressed to: Romy Campisano National Homeland Security Research Center Office of Research and Development (NG16) U.S. Environmental Protection Agency 26 West Martin Luther King Drive Cincinnati, OH 45268 (513) 569-7016 [email protected] Kathy Hall National Homeland Security Research Center Office of Research and Development (NG16) U.S. Environmental Protection Agency 26 West Martin Luther King -

Laboratory Equipment AP
\ \\ , f ?7-\ Watch glass 1 Crucible and cover Evaporating dish Pneumatlo trough Beaker Safety goggles Florence Wide-mouth0 Plastic wash Dropper Funnel flask collecting bottle pipet Edenmeyer Rubber stoppers bottle flask € ....... ">. ÿ ,, Glass rod with niohrome wire Scoopula (for flame re,sting) CruoiNe tongs Rubber ubing '1 ,v .... Test-tube brush square Wire gau ÿ "\ file Burner " Tripod Florence flask: glass; common sizes are 125 mL, 250 mL, 500 .d Beaker: glass or plastic; common sizes are 50 mL, mL; maybe heated; used in making and for storing solutions. 100 mL, 250 mL, 400 mL; glass beakers maybe heated. oÿ Buret: glass; common sizes are 25 mL and 50 mL; used to Forceps: metal; used to hold or pick up small objects. Funnel: glass or plastic; common size holds 12.5-cm diameter measure volumes of solutions in titrafions. Ceramic square: used under hot apparatus or glassware. filter paper. Gas burner: constructed of metal; connected to a gas supply Clamps" the following types of clamps may be fastened to with rubber tubing; used to heat chemicals (dry or in solution) support apparatus: buret/test-tube clamp, clamp holder, double buret clamp, ring clamp, 3-pronged jaw clamp. in beakers, test tubes, and crucibles. Gas collecting tube: glass; marked in mL intervals; used to 3: Clay triangle: wire frame with porcelain supports; used to o} support a crucible. measure gas volumes. Glass rod with nichrome wire: used in flame tests. Condenser: glass; used in distillation procedures. Q. Crucible and cover: porcelain; used to heat small amounts of Graduated cylinder: glass or plastic; common sizes are 10 mL, 50 mL, 100 mL; used to measure approximate volumes; must solid substances at high temperatures. -

Lab # 4: Separation of a Mixture
Name: Date Completed: Lab Partner(s): Lab # 4: Separation of a Mixture Lab Accelerated Chemistry 1 Objective You will be given a mixture containing sodium chloride (NaCl, table salt), benzoic acid (C7H6O2, a common food preservative), and silicon dioxide (SiO2, sand). Your goal is to separate the substances and determine the percent of each in the original mixture. Here is the separation scheme: Mixture (NaCl, benzoic acid, sand) Extract with 100°C H O 2 Decant residue supernatant Sand (wet) NaCl, benzoic acid Cool Filter evaporate H O 2 residue filtrate Sand (dry) Benzoic acid (wet) NaCl (solution) evaporate H2O Benzoic acid (dry) Introduction Mixtures are not unique to chemistry; we use and consume them on a daily basis. The beverages we drink each morning, the fuel we use in our automobiles, and the ground we walk on are mixtures. Very few materials we encounter are pure. Any material made up of two or more substances that are not chemically combined is a mixture. The isolation of pure components of a mixture requires the separation of one component from another. Chemists have developed techniques for doing this. These methods take advantage of the differences in physical properties of the components. The techniques to be demonstrated in this laboratory are the following: Extraction. This uses a solvent to selectively dissolve one component of the solid mixture. With this technique, a soluble solid can be separated from an insoluble solid. Decantation. This separates a liquid from insoluble solid sediment by carefully pouring the liquid from the solid without disturbing the solid. Recrystallization. -

Human Leukemia T-Cell Lines As Alternatives to Animal Use for Detecting Biologically Active Staphylococcal Enterotoxin Type B
toxins Article Human Leukemia T-Cell Lines as Alternatives to Animal Use for Detecting Biologically Active Staphylococcal Enterotoxin Type B Reuven Rasooly *, Paula Do , Xiaohua He and Bradley Hernlem Foodborne Toxin Detection & Prevention Research Unit, Western Regional Research Center, Agricultural Research Service, United States Department of Agriculture, Albany, CA 94710, USA; [email protected] (P.D.); [email protected] (X.H.); [email protected] (B.H.) * Correspondence: [email protected]; Tel.: +1-510-559-6478 Abstract: Staphylococcal enterotoxin type B (SEB) is associated with food poisoning. Current methods for the detection of biologically active SEB rely upon its ability to cause emesis when administered to live kittens or monkeys. This technique suffers from poor reproducibility and low sensitivity and is ethically disfavored over concerns for the welfare of laboratory animals. The data presented here show the first successful implementation of an alternative method to live animal testing that utilizes SEB super-antigenic activity to induce cytokine production for specific novel cell-based assays for quantifiable detection of active SEB. Rather than using or sacrificing live animals, we found that SEB can bind to the major histocompatibility complex (MHC) class II molecules on Raji B-cells. We presented this SEB–MHC class II complex to specific Vβ5.3 regions of the human T-cell line HPB-ALL, which led to a dose-dependent secretion of IL-2 that is capable of being quantified and can further detect 10 pg/mL of SEB. This new assay is 100,000 times more sensitive than the ex vivo murine splenocyte method that achieved a detection limit of 1 µg/mL. -

Development of Real-Time PCR Screening Methods for the Detection of Bacterial Toxin DNA
Development of Real-time PCR Screening Methods for the Detection of Bacterial Toxin DNA Christina Egan, Ph.D. Director, Biodefense Laboratory Wadsworth Center N New York State Dept. of Health Y S D O [email protected] H Biodefense Wadsworth Center Laboratory New York State Department of Health Overview • Wadsworth Center Biodefense Laboratory • Utility of rtPCR as a rapid screening method • Staphylococcal enterotoxin rtPCR • Clostridium botulinum rtPCR • New and upcoming technology N Y S D O H Biodefense Wadsworth Center Laboratory New York State Department of Health Biodefense Laboratory Services Threats Training •Powders, letters, “All Hazards” •Laboratorians •Biohazard Detection System •First Responders Foods •Intentional/Unintentional Assay Development Animals •Emerging diseases •Novel technologies •Wild, domestic •Optimized methods •Outbreaks, investigations Patients Network Partnerships •Naturally occuring •LRN •Smallpox vs. Vaccination events •FERN N Y •Emerging Diseases (SARS, Flu) •ERLN, ECLRN S D •USPS, BDS O H Biodefense Wadsworth Center Laboratory New York State Department of Health rtPCR Assay Development • Follow standardized procedure in the laboratory, based on guidelines developed for clinical specimen testing • Distinct development and validation phase • Important to evaluate extraction and mastermix components of assay • Assays were designed with multiplexing capability in mind • Assays are validated as singleplex assays and evaluated to meet analytical criteria (sensitivity, N Y specificity, etc.) and then diagnostic -

Used for Moving Beakers Off of Hot Surfaces
Lab Equipment and Use Review Sheet Glassware Function Glassware Function Glassware Function Running Accurately Running reactions, measuring/ reactions, heating chemicals mixing mixing delivering chemicals – chemicals, volumes of easier for mixing Beaker heating Buret liquids than beakers chemicals Erlenmeyer Flask Used in Running Used to mix vacuum reactions, chemicals to heating filtration chemicals accurately mixing determine chemicals – concentration; Volumetric easier for contains exact Florence Flask Flask Filter Flask mixing than volumes beakers Used for Used for Used for mixing filtering and accurately and heating chemicals and for adding measuring running chemicals the volume reactions– without of liquids. smaller quantities Funnel Graduated spilling Test Tube than beakers and Cylinder flasks. Holding For stirring For storing Chemicals, Glass Stirring Rod chemicals small amounts covering of chemicals Watch Glass beakers during Sample Vial heating For adding Used to small evaporate amounts of Used for Evaporating liquids chemicals – lighting burner Dish usually by Plastic Pipets drops Used to add Utility Clamp – deionized used to hold water; to add objects on a solvents for ring stand. cleaning of Iron ring – Squirt Bottle beakers and used to hold other objects above glassware. a Bunsen burner flame. Ring Stand Ring stand – Beaker Tongs with Utility used to hold Clamp and various Iron Ring objects. Equipment Function Equipment Function Equipment Function Heat source in Used to grind Used to clean the chemistry chemicals into glassware lab. Uses powder natural gas. Mortar and Pestle Test tube brush/beaker Bunsen Burner brush Used to hold a Used to Used to crucible above transport hot strongly heat a flame – used Crucible tongs crucibles and substances Clay triangle in conjunction to remove Crucible and above a flame with iron ring their covers Cover Used to hold Used to handle a Used in group of test single test tube.