Lab # 4: Separation of a Mixture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VACUUM RECOVERY of ASPHALT EMULSION RESIDUE (An Arizona Method)
ARIZ 504 July 1980 (3 Pages) VACUUM RECOVERY OF ASPHALT EMULSION RESIDUE (An Arizona Method) Scope (d) No. 10 sieve conforming to AASHTO designation M 92. I. This method describes a low temperature vacuum procedure for recovery of the asphalt residue (e) Vacuum recovery apparatus as shown from asphalt emulsions. It is is not suitable for assembled in Fig. I. quantitative recovery of solvents from emulsions I) Vacuum source capable of producing an containing low boiling range distillates. absolute vacuum within the system of approximately 710 mm (28 in.) mercury. Apparatus 2) Thermometer - shall have a range of _5° to 1. The apparatus shall consist of the following: +200°C (23°F to 392°F). The overall length (a) Brass stirring rod. shall be 600 mm (24 in.) and the distance from the bottom of the bulb to the zero point (b) 8 oz. ointment can. shall be 300 mm (12 in.) (c) 100 ml. stainless steel beaker. 3) Stirrer hot plate. VACUUM RECOVERY APPARATUS 300 mm Allihn condenser H20 Out / Thermometer Vacuum Release Pinch Clamp 500 m. 1,000 ml. Filtration Flask Filtration Flask Teflon Stirring Bar H20 In 500 ml. Filtration Flask Portable Heat Gun FIGURE I ARIZ 504 July 1980 4) Teflon covered stirring bar. (g) Insert the stoppered themometer (positioned 5) 1000 ml. and two 500 ml. filtering flasks with in the stopper at an angle to prevent contact with tubulation. stirring bar) into the flask and set on hot plate at a medium high heat setting (#4). The bulb of the 6) 300 mm Allihn condensor. -

LAB: One Tube Reaction Part 1
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube, iron nail, sodium chloride, copper (II) sulfate, distilled water, glass stirring rod, tissue paper, parafilm wax Procedures: 1. Label a test tube to identify your group 2. Fill approx. 1/3 of the test tube with copper (II) sulfate crystals. Gently tap the tube to allow the crystals to settle. 3. Using a glass stirring rod, carefully cover the crystals with a layer of Kimwipe tissue paper. 4. Slowly, and with as little disturbance as possible, add enough distilled water to just cover the paper and blue crystals. 5. Repeat the process for the sodium chloride, filling approx. 1/3 of the test tube. Gently tap the tube to allow the crystals to settle. 6. Push more tissue paper into the test tube on top of the white crystals. 7. Add enough water to cover the tissue paper and white crystals. 8. Obtain an iron nail and expose the surface by rubbing with sand paper. 9. Carefully slide the nail into the test tube. 10. Continue adding water until the nail is completely covered. 11. Cover the test tube with parafilm wax and record your Day 1 observations. 12. Return the test tube to the test tube rack. 13. Continue to monitor and record your observations for the next several days, as indicated in the data table. Data: 1. Complete the NFPA label for the two chemicals used in this lab. -

Equipment Detailsr07
Lab Equipment Details Lab Equipment Glass Flasks 150ml 250ml 500ml Lab Equipment Glass Beakers 150ml 250ml 500ml Lab Equipment Glassware But once removed, only the cap stays highlighted. Droppers critical to Lab An activated Dropper coursework can be found highlights the entire bottle. already in the workspace. Dropper Dropper Dropper Activated In Use Lab Equipment Gastight Syringe Small A pre-filled gastight syringe can be used with a NMR tube to safely fill through the top in preparation for use with the NMR spectrometer. Gastight Syringe NMR Tube with Holder with Holder Lab Equipment NMR Tube Spinner An NMR Tube filled with gas for use with the NMR Spectrometer Simply use the NMR Tube with needs to be inserted into the the Tube Spinner. Spinner before it can be used. NMR Tube NMR Tube Spinner NMR Tube Spinner with Holder with NMR Tube inserted Once the Tube slotted into the holder is inserted into the top part of the spectrometer, users can type in a number for Lab Equipment the frequency they’d like to scan. NMR Spectrometer XL The NMR Tube holder can then be slotted into the highlighted tube on the NMR Spectrometer. NMR Tube filled with appropriate substance is slotted (used with) in the holder. Lift will perpare the Spectrometer for the tube holder insertion. Scan No. Allows the user to change the frequency at which the tube is scanned The NMR Tube holder can then be slotted into the top tube on Lab Equipment the NMR Spectrometer. NMR Spectrometer XL NMR Tube filled with appropriate substance is slotted (used with) in the holder. -

METHODS & STANDARD OPERATING PROCEDURES (Sops)
METHODS & STANDARD OPERATING PROCEDURES (SOPs) OF EMISSION TESTING IN HAZARDOUS WASTE INCINERATOR FOREWARD COVR PAGE TEAM CHAPTER TITLE PAGE NO Chapter - 1 Stack Monitoring – Material And Methodology For Isokinetic Sampling 1-28 Chapter - 2 Standard Operating Procedure (SOP) For Particulate Matter Determination 29-38 Chapter - 3 Determination Of Hydrogen Halides (Hx) And Halogens From Source Emission 39-54 Chapter - 4 Standard Operating Procedure For The Sampling Of Hydrogen Halides And Halogens From Source Emission 55-61 Determination Of Metals And Non Metals Emissions From Chapter - 5 Stationarysources 62-79 Standard Operating Procedure (Sop) For Sampling Of Metals And Non Chapter - 6 Metals & 80-97 Standard Operating Procedure (Sop) Of Sample Preparation For Analysis Of Metals And Non Metals Chapter - 7 Determination Of Polychlorinated Dibenzo-P-Dioxins And 98-127 Polychlorinated Dibenzofurans Standard Operating Procedure For Sampling Of Polychlorinated Chapter - 8 Dibenzo-P-Dioxins And Polychlorinated Dibenzofurans & 128-156 Standard Operating Procedure For Analysis Of Polychlorinated Dibenzo- P-Dioxins And Polychlorinated Dibenzofurans Project Team 1. Dr. B. Sengupta, Member Secretary - Overall supervision 2. Dr. S.D. Makhijani, Director - Report Editing 3. Sh. N.K. Verma, Ex Additional Director - Project co-ordination 4. Sh. P.M. Ansari, Additional Director - Report Finalisation 5. Dr. D.D. Basu, Senior Scientist - Guidance and supervision 6. Sh. Paritosh Kumar, Sr. Env. Engineer - Report processing 7. Sh. Dinabandhu Gouda, Env. Engineer - Data compilation, analysis and preparation of final report 8. Sh. G. Thirumurthy, AEE - - do - 9. Sh. Abhijit Pathak, SSA - - do - 10. Ms. Trapti Dubey, Jr. Professional (Scientist) - - do - 11. Er. Purushotham, Jr. Professional (Engineer) - - do - 12. -

Catching up with Runaway Hot Plates
Catching up with Runaway Hot Plates Kimberly Brown1, Mark Mathews2, Joseph Pickel3 1‐ Office of Environmental Health and Radiation Safety University of Pennsylvania, Philadelphia, PA 2‐ Environmental Safety and Health Directorate, Oak Ridge National Laboratory, Oak Ridge TN 3‐ Physical Sciences Directorate, Oak Ridge National Laboratory, Oak Ridge TN Recent Events with Malfunctioning Abstract Cause of Fire identified as Runaway Hot Plates Hotplate Several research institutions report safety events involving hotplates that have been In recent years, there have been numerous reports of runaway hot plates — hot left on and unattended or that have heated uncontrollably, sometimes resulting in plates that heat uncontrolled despite the setting or the fact that the controls are in significant damage in the process. Some of the known events are listed below: the off position. Some of these events have resulted in damage to research • June 29, 2005: Lawrence Berkeley National Laboratory — A laboratory hot plate’s facilities. The Division of Chemical Health and Safety has investigated this issue power switch failed, resulting in a fire in the fume hood. Although the switch using all available information and soliciting additional information viaasurvey position, switch detent, and indicator light demonstrated that the power was off, conducted in April 2017 to determine the cause of these issues and to develop Valid NRTL listing electrical power continued to flow to the heating elements. strategies to prevent future “runaways‘’. Results of this survey and best practices (http://www2.lbl.gov/ehs/Lessons/pdf/FinalHotPlateLL.pdf) are described herein. • April 2007: University of California — Following a March 2007 report of a malfunctioning hot plate that heats while in the ‘off’ position, UC EHS personnel send out a system wide message concerning the issue citing “several explosions or Survey Information fire events involving defective hot plates that resulted in injuries to laboratory employees or damage to laboratories” over the previous five years. -

Science Safety
2010 Mississippi Science Framework Science Safety The guides that are cited below were developed by the Council of State Science Supervisors (CSSS) with support from the Eisenhower National Clearinghouse for Mathematics and Science Education, the National Aeronautics and Space Administration, Dupont Corporation, Intel Corporation, Americal Chemical Society, and the National Institutes of Health. Science Safety Booklets may be printed for use by educators. • Science Safety Booklets • Science and Safety: It's Elementary (PDF) - A Elementary Safety Guide • Science and Safety, Making the Connection (PDF) - A Secondary Safety Guide . Approved July 25, 2008 1 2010 Mississippi Science Framework A SUGGESTED PATTERN FOR CHEMICAL STORAGE The alphabetical method for storing chemicals presents hazards because chemicals, which can react violently with each other, may be stored in close proximity. Schools may wish to devise a simple color-coding scheme to address this problem. The code shown below, reproduced with permission from School Science Laboratories-A Guide to Some Hazardous Substances by the Council of State Science Supervisors, includes both solid and striped colors which are used to designate specific hazards as follows: Red - Flammability hazard: Store in a flammable chemical storage area. Red Stripe - Flammability hazard: Do not store in the same area as other flammable substances. Yellow - Reactivity hazard: Store separately from other chemicals. Yellow Stripe - Reactivity hazard: Do not store with other yellow coded chemicals; store separately. White - Contact hazard: Store separately in a corrosion-proof container. White Stripe - Contact hazard: Not compatible with chemicals in solid white category. Blue - Health hazard: Store in a secure poison area. Orange - Not suitably characterized by any of the foregoing categories. -

Documm Mgson!
Documm mgson! ED 174 412 SE 027 981 TITLE Biomedical Science, Unit I: Respiration inHealth and Medicine. Respiratory Anatomy, Physiology and Pathology; The Behavior of Gases;Introductory Chemistry; and Air Pollution. Laboratory Manual. Revised Version, 1975. INSTITUTION Biomedical Interdisciplinary Curriculum Project, Berkeley, Calif. SPONS AGENCY National Science Foundation, Washington, D.C. PUB DATE 75 NOTE 181p.; For related documents, see SE 027978-999 and SE 028 510-516; Not available in hard copydue to copyright restrictions EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIpTCRS Chemistry; Environment; Health; *HealthEducation; Higher Education; *Laboratory ExFeriments; *Laboratory Techniques; *Laboratory Training;Medical Education; Pathology; Physics; *Physiology;*Science Education; Secondary Education IDENTIFIERS *Respiration ABSTItACT Designed to accompany the student text on respiration, this manual presents instructions on the use of labcra tcry equipment and presents various experimentsdealing with the concepts presented in the text. Thirty=nine laboratoryactivities ' are de scribed. Laboratory activities are dividedinto several parts, each Part covering a specific experiment dealing with the concept covered by the activity. Each experimentincludes a description of materials, procedures, and discurision questions. (RE) ********************************************************************** Reproductions supplied by EDRS are the bestthat can be made from the original document. 4********************M414141#***********4141*****4141***********41#********4 -

Information to Users
INFORMATION TO USERS While the most advanced technology has been used to photograph and reproduce this manuscript, the quality of the reproduction is heavily dependent upon the quality of the material submitted. For example: # Manuscript pages may have indistinct print. In such cases, the best available copy has been filmed. ® Manuscripts may not always be complete. In such cases, a note will indicate that it is not possible to obtain missing pages. ® Copyrighted material may have been removed from the manuscript. In such cases, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, and charts) are photographed by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each oversize page is also filmed as one exposure and is available, for an additional charge, as a standard 35mm slide or as a 17”x 23" black and white photographic print. Most photographs reproduce acceptably on positive microfilm or microfiche but lack the clarity on xerographic copies made from the microfilm. For an additional charge, 35mm slides of 6”x 9” black and white photographic prints are available for any photographs or illustrations that cannot be reproduced satisfactorily by xerography. Order Number 8717718 On-line, adaptive, optimal control of a high-density, fed-batch fermentation of streptomyces C5 Schlasner, Steven Mark, Ph.D. The Ohio State University, 1987 UMI 300N.ZeebRd. Ann Arbor, MI 48106 PLEASE NOTE: In all cases this material has been filmed in the best possible way from the available copy. Problems encountered with this document have been identified here with a check mark V 1. -

Vworks Automation Control User Guide
VWorks Automation Control User Guide Original Instruction Agilent Technologies Notices © Agilent Technologies, Inc. 2010 Warranty (June1987) or DFAR 252.227-7015 (b)(2) (November 1995), as applicable in any No part of this manual may be reproduced The material contained in this docu- technical data. in any form or by any means (including ment is provided “as is,” and is sub- electronic storage and retrieval or transla- ject to being changed, without notice, Safety Noticies tion into a foreign language) without prior agreement and written consent from Agi- in future editions. Further, to the max- A WARNING notice denotes a lent Technologies, Inc. as governed by imum extent permitted by applicable hazard. It calls attention to an United States and international copyright law, Agilent disclaims all warranties, operating procedure, practice, or the laws. either express or implied, with regard like that, if not correctly performed or to this manual and any information User Guide Part Number adhered to, could result in personal contained herein, including but not injury or death. Do not proceed G5415-90063 limited to the implied warranties of beyond a WARNING notice until the merchantability and fitness for a par- indicated conditions are fully Edition ticular purpose. Agilent shall not be understood and met. liable for errors or for incidental or Revision 01, April 2010 consequential damages in connection A CAUTION notice denotes a hazard. It Technical Support with the furnishing, use, or perfor- calls attention to an operating procedure, mance of this document or of any practice, or the like that, if not correctly performed or adhered to, could result in Agilent Technologies Inc. -

Hot Plate Safety
LABORATORY SAFETY GUIDELINE Hot Plate Safety Hot plates used in labs present many potential dangers, such as burns, fires, and electrical shock, which can cause injuries, significant disruption of lab operations, and loss of scientific data. Burns • The hot plate is a source of heat when on, and for some time after it has been turned off. The temperature of the heated plate can reach 500°C and can cause severe burns. Electric Shock • Touching the hot plate with the power cords can melt through the insultation and cause electric shock. Fire Hazard • Electrical spark hazard from either the on-off switch or the bimetallic thermostat used to regulate temperature, or both, are a design flaw issue in older hotplate models. If the equipment sparks near combustible or flammable materials, fire could result. • Exercise caution when heating flammable materials. • Hot/stirrer plates have an additional risk if an operator accidentally turns on the wrong feature. • Hot plates are NOT explosion proof or intrinsically safe. Basic Precautions: • Only use hot plates that have been approved by a Nationally Recognized Testing Laboratory (NRTL) such as Underwriter’s Laboratory (UL). • Read the manufacturer’s instructions before using and consider registering the device with the manufacturer so you will be notified of any warnings or recalls. • Periodically inspect equipment prior to use: o Do not use if the plug or cord is worn, frayed, or damaged, if the grounding pin has been removed, or if a spark is observed. o Check for corrosion of the thermostat, which can cause a spark. o Test the function of the “off” switch on each hot plate to verify that it works and the device cools quickly when the switch is in the “off” position. -

Process Skills Review Warm-Up C
Process Skills Review Warm-Up C. • Define function • Match the following 1. Puts out fire 2. Curved line of liquid in a graduated cylinder 3. Used to observe insects A. B. Which of the following describes the correct way to handle chemicals in a laboratory? A. It is safe to combine unknown chemicals as long as only small amounts are used. B. Return all chemicals to their original containers. C. Always pour extra amounts of the chemicals called for the experiment. D. To test for odors, always use a wafting motion. Which of the following describes the correct way to handle chemicals in a laboratory? A. It is safe to combine unknown chemicals as long as only small amounts are used. B. Return all chemicals to their original containers. C. Always pour extra amounts of the chemicals called for the experiment. D. To test for odors, always use a wafting motion. What task is being performed in the picture? A. measuring mass with a graduated cylinder B. measuring length with a triple beam balance C. measuring mass with a triple beam balance D. measuring length with a metric ruler What task is being performed in the picture? A. measuring mass with a graduated cylinder B. measuring length with a triple beam balance C. measuring mass with a triple beam balance D. measuring length with a metric ruler John and Lisa are conducting an experiment in their 7th grade science class in which they are handling potentially dangerous chemicals. As John is pouring a chemical from a beaker to a graduated cylinder, he splashes some of the chemical into his eyes. -
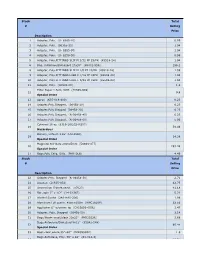
Stock Total # Selling Price 1 Adapter, Poly. (U- 6365-10)
Stock Total # Selling Price Description 1 Adapter, Poly. (U- 6365-10) 0.99 2 Adapter, Poly. (06365-22) 1.04 3 Adapter, Poly. (U- 6365-30) 1.04 4 Adapter, Poly. (U- 6359-50) 0.99 5 Adapter, Poly,FITTINGS SLIP M 3/32 PP 25/PK (45518-24) 1.04 6 Bag, Autoclave/Biohazard 24x30'' (95042-556) 156.1 8 Adapter, Poly.FITTINGS SLIP M 1/8 PP 25/PK (45518-26) 1.59 9 Adapter, Poly.FITTINGS LUER F 1/16 PP 25PK (45508-00) 1.04 10 Adapter, Poly.FITTINGS LUER F 3/32 PP 25PK (45508-02) 1.04 11 Adapter, Poly. (30800-08) 1.3 Filter Paper 1.5cm, VWR (28309-989) 12 9.9 Special Order 13 Apron (635-018-400) 0.23 14 Adapter,Poly.Stepped, (06458-10) 6.23 15 Adapter,Poly.Stepped (06458-20) 6.23 16 Adapter,Poly.Stepped, (N-06458-40) 6.23 17 Adapter,Poly.Stepped, (N-06458-60) 5.06 Cytoseal 16 oz (8310-16/23244257) 18 58.49 Hazardous Battery, Lithium 3.6V (LS14500) 19 14.38 Special Order Magnetic Stir Bars 2mmx5mm (58948-377) 20 293.48 Special Order 21 Bags,Poly Cello,10 lb. (PKR10Lb) 4.49 Stock Total # Selling Price Description 22 Adapter,Poly. Stepped (N-06458-30) 2.71 24 Alconox (21835-032) 42.75 25 Ammonium Sulfate,certif. (A7023) 41.18 26 Bar, spin 2'' x 1/2'' (14-51367) 5.22 27 Alcohol Swabs (248-HAS-200) 1.96 28 Aluminum Foil comm. 45cmx100m (WPC1810P) 32.56 29 Applicator 6'' w/cotton tip (CA10806-000L) 1.47 30 Adapter, Poly.