Corning's Care and Safe Handling of Glassware Application Note
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VACUUM RECOVERY of ASPHALT EMULSION RESIDUE (An Arizona Method)
ARIZ 504 July 1980 (3 Pages) VACUUM RECOVERY OF ASPHALT EMULSION RESIDUE (An Arizona Method) Scope (d) No. 10 sieve conforming to AASHTO designation M 92. I. This method describes a low temperature vacuum procedure for recovery of the asphalt residue (e) Vacuum recovery apparatus as shown from asphalt emulsions. It is is not suitable for assembled in Fig. I. quantitative recovery of solvents from emulsions I) Vacuum source capable of producing an containing low boiling range distillates. absolute vacuum within the system of approximately 710 mm (28 in.) mercury. Apparatus 2) Thermometer - shall have a range of _5° to 1. The apparatus shall consist of the following: +200°C (23°F to 392°F). The overall length (a) Brass stirring rod. shall be 600 mm (24 in.) and the distance from the bottom of the bulb to the zero point (b) 8 oz. ointment can. shall be 300 mm (12 in.) (c) 100 ml. stainless steel beaker. 3) Stirrer hot plate. VACUUM RECOVERY APPARATUS 300 mm Allihn condenser H20 Out / Thermometer Vacuum Release Pinch Clamp 500 m. 1,000 ml. Filtration Flask Filtration Flask Teflon Stirring Bar H20 In 500 ml. Filtration Flask Portable Heat Gun FIGURE I ARIZ 504 July 1980 4) Teflon covered stirring bar. (g) Insert the stoppered themometer (positioned 5) 1000 ml. and two 500 ml. filtering flasks with in the stopper at an angle to prevent contact with tubulation. stirring bar) into the flask and set on hot plate at a medium high heat setting (#4). The bulb of the 6) 300 mm Allihn condensor. -

CASE 6 Shelf 1 #1 Fancy Opaque Glass 1982.65 WATER Challinor Taylor and Paneled Flower Funds Provided PITCHER Co., Pittsburgh, PA, Pattern, No
CASE 6 Shelf 1 #1 Fancy Opaque Glass 1982.65 WATER Challinor Taylor and Paneled Flower Funds provided PITCHER Co., Pittsburgh, PA, pattern, No. 23, by the Fifth patent June 1, 1886 pressed purple slag Annual Benefit opaque soda-lime Antiques Show glass Shelf 1 #2 Fancy Opaque Glass 1990.78 ELECTRIC New Martinsville Peachblow or Gift of Mrs. LIGHT Glass Mfg. Co., New Sunburst line, shaded Betty Woods SHADE Martinsville, WV, pink mold-blown Daniel about 1905-1910 glass with iridescent gold lining 1982.183AB BRIDE’S New Martinsville New Martinsville Gift of Mrs. BOWL ON Glass Mfg. Co., New Peachblow, pink Betty Woods STAND Martinsville, WV, shaded to white Daniel about 1905-1910 mold-blown glass, silver plated stand Shelf 1 #3 Opalescent and Iridescent 1981.138 BERRY Northwood Glass Diamond Funds provided BOWL Company, Indiana, Spearhead pattern, by Mr. Arthur B. PA, about 1900 deep blue shaded to Beaumont opalescent pressed glass 1990.109.1 TWO Dugan Glass Co., Diamond Museum 1990.109.2 MATCHING Indiana, PA, about Spearhead pattern, purchase INDIVIDUAL 1910 deep blue shaded to BERRY opalescent pressed BOWLS glass Shelf 1 #4 Opalescent and Iridescent 1000.164 DISH Dugan Glass Co., Peach opalescent Museum Indiana, PA, about pressed soda-lime purchase 1910-1914 glass with iridescent finish CASE 6 - Page 1 1989.72 BOWL Dugan Glass Co., Petals and Fans Museum Indiana, PA, about pattern on front with purchase 1910-1914 Jeweled Heart pattern on back, amethyst pressed soda-lime glass with deep iridescent finish 1994.39 PLATE Dugan Glass Co., Persian -

Tevilas Keilim
STAR-K Kosher Classroom HALACHOS OF TEVIFOOD UTENSIL TEVILA GUIDELINELASFOOD UTENSIL TEVILAKEILIM GUIDELINE FOOD UTENSIL TEVILA GUIDELINE Earthenware, Non-Glazed Porcelain Enamel Tevila w/o Brocha Aluminum Pans, Disposable Tevila with Brocha No Tevila to be used more than once Dull Finish, e.g. Flower Pot Racks, Cooling Tevila w/o Brocha George Foreman Grill Tevila w/o Brocha Aluminum Pans, Disposable Tevila w/o Brocha Racks, Oven No Tevila to be used only once (including Pyrex, Duralex & Corelle) Glass Tevila with Brocha Rolling Pins Metal or Wood No Tevila Blech No Tevila Hot Air Popcorn Maker, Metal Tevila with Brocha Sandwich Maker Tevila w/o Brocha Blender -Handheld Immersion Hot Water Urn, Metal Tevila with Brocha Wand with metal blade on bottom Tevila with Brocha Sink Racks, Stainless Steel No Tevila Ice Cream Scooper Tevila with Brocha Bone No Tevila Spatula, Metal Tevila with Brocha Knife, Arts & Crafts No Tevila Brush, Pastry No Tevila Stoneware Tevila w/o Brocha Knife Sharpener No Tevila Brush for Grill, Metal No Tevila Stoneware, Non-Glazed No Tevila Meat Thermometer No Tevila Can Opener No Tevila Storage Utensils, Glass Meat Tenderizer Hammer, not brought to the table No Tevila Cans, Reusable Empty Metal No Tevila if opened by a Yehudi No Tevila Metal Cutlery Tevila with Brocha Styrofoam No Tevila China, Bone Tevila w/o Brocha Tea Kettle, Corelle Tevila with Brocha Metal Flour & Sugar Storage Tevila w/o Brocha China, Glazed Tevila w/o Brocha Canisters Thermos (Glass Insert) Tevila with Brocha Colander, Metal Tevila with Brocha -

Set up for Heating Kcl + Kcloз
Set Up for heating KCl + KClOз Crucible with unknown mixture plus MnO₂ Extension clamp Bunsen Burner To Gas Line Set Up for heating KCl + KClOз by Ashley Kajioka is licensed under a Creative Commons Attribution 4.0 International License. Procedure: Obtain a porcelain crucible, crucible lid, and a clay triangle. Clean the crucible, removing any loose particulate matter, and check the crucible for cracks. It is not necessary to remove all debris from the crucible as most of it has been fused to the porcelain and cannot be removed. In you fume hood, set up the apparatus as shown in Figure 5. a . b. e. Figure 5: Crucible setup d. a. Ring stand b. Crucible lid c. Iron ring d. Clay triangle c. e. Crucible “Lab Instructions” by Ashley Kajioka is licensed under CC BY 4.0 Using your crucible tongs, practice the following techniques on the cool crucible: Lifting the lid from the crucible Placing the lid ajar so that the crucible is slightly open, but the lid will not fall off, as in Figure 5. Quickly, but gently, pushing the lid from its ajar position to completely cover the crucible Lifting the crucible, with its lid, from the clay triangle Carrying the crucible and lid while supporting from underneath with your wire gauze. Practicing these techniques may seem silly, but it is worth your time to do so. Would you rather repeat a trial because you dropped a crucible, shattering it into a million pieces? With your covered crucible in the clay triangle, fire the crucible for 3-5 minutes. -
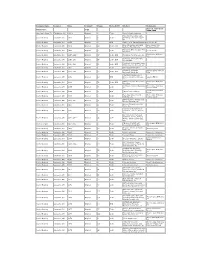
Catalog Holding List A-E in PDF Form
Company Name Location Date Original? Pages Color-B/W? Product Comments See U.S. Glass Circa Adams & Co. Copy 1890-1900 Akro Agate Glass Co. Clarksburg, WV 1940’s Original 4 Color Akroite floral containers Decorated beverage ware, Anchor Hocking Lancaster, OH 1942 Original 11 Color Fire-King oven ware & jade- ite Anchor Hocking Lancaster, OH 1953 Original 4 B/W Cape Cod or Sandwich pattern Loose sheets Decorated glass, Sandwich Some pages have Anchor Hocking Lancaster, OH 1954 Original 44 Color, B/W pattern, Fire-King, etc. pictures cut out Turquoise Blue & Copper Tint Anchor Hocking Lancaster, OH 1956 Original 5 Color Loose sheets Fire-King Plus 4 page B/W price Anchor Hocking Lancaster, OH 1957-1958 Original 47 Color, B/W Fire-King, Forest Green, etc. list Fire-King, beverage items, Anchor Hocking Lancaster, OH 1959-1960 Original 56 Color, B/W kitchenware Fire-King, kitchenware, some Anchor Hocking Lancaster, OH 1960-1961 Original 59 Color, B/W Early American Pres-cut, etc. Anchor Hocking Lancaster, OH 1961 Original 1 Color Desert gold stemware Fire-King, Sandwich, Several pages with cut Anchor Hocking Lancaster, OH 1961-1962 Original 67 Color, B/W beverage items, etc. outs “Institutional Glassware”, Anchor Hocking Lancaster, OH 1963 Original 12 B/W Stems, tumblers, Ashtrays, Catalog HR 63 etc. Mostly beverage ware, some Plus 8 page B/W price Anchor Hocking Lancaster, OH 1964 Original 78 Color, B/W Fire-King list Tumblers, Prescut, Ovenware, Plus 2 page B/W price Anchor Hocking Lancaster, OH 1965 Original 78 Color etc. revision sheet Loose sheet, includes Anchor Hocking Lancaster, OH 1965 Original 2 B/W “Bravo” Cut Tumblers prices Beverage Ware, Prescut, , Plus 1 page B/W price Anchor Hocking Lancaster, OH 1966 Original 80 Color Jade-ite, etc. -

METHODS & STANDARD OPERATING PROCEDURES (Sops)
METHODS & STANDARD OPERATING PROCEDURES (SOPs) OF EMISSION TESTING IN HAZARDOUS WASTE INCINERATOR FOREWARD COVR PAGE TEAM CHAPTER TITLE PAGE NO Chapter - 1 Stack Monitoring – Material And Methodology For Isokinetic Sampling 1-28 Chapter - 2 Standard Operating Procedure (SOP) For Particulate Matter Determination 29-38 Chapter - 3 Determination Of Hydrogen Halides (Hx) And Halogens From Source Emission 39-54 Chapter - 4 Standard Operating Procedure For The Sampling Of Hydrogen Halides And Halogens From Source Emission 55-61 Determination Of Metals And Non Metals Emissions From Chapter - 5 Stationarysources 62-79 Standard Operating Procedure (Sop) For Sampling Of Metals And Non Chapter - 6 Metals & 80-97 Standard Operating Procedure (Sop) Of Sample Preparation For Analysis Of Metals And Non Metals Chapter - 7 Determination Of Polychlorinated Dibenzo-P-Dioxins And 98-127 Polychlorinated Dibenzofurans Standard Operating Procedure For Sampling Of Polychlorinated Chapter - 8 Dibenzo-P-Dioxins And Polychlorinated Dibenzofurans & 128-156 Standard Operating Procedure For Analysis Of Polychlorinated Dibenzo- P-Dioxins And Polychlorinated Dibenzofurans Project Team 1. Dr. B. Sengupta, Member Secretary - Overall supervision 2. Dr. S.D. Makhijani, Director - Report Editing 3. Sh. N.K. Verma, Ex Additional Director - Project co-ordination 4. Sh. P.M. Ansari, Additional Director - Report Finalisation 5. Dr. D.D. Basu, Senior Scientist - Guidance and supervision 6. Sh. Paritosh Kumar, Sr. Env. Engineer - Report processing 7. Sh. Dinabandhu Gouda, Env. Engineer - Data compilation, analysis and preparation of final report 8. Sh. G. Thirumurthy, AEE - - do - 9. Sh. Abhijit Pathak, SSA - - do - 10. Ms. Trapti Dubey, Jr. Professional (Scientist) - - do - 11. Er. Purushotham, Jr. Professional (Engineer) - - do - 12. -

Glossary of Terms Abrisa Technologies Your Single Source Optics Partner!
Glossary of Terms Abrisa Technologies Your Single Source Optics Partner! December 2015 200 South Hallock Drive, Santa Paula, CA 93060 • (877) 622-7472 • FAX (805) 525-8604 • www.abrisatechnologies.com Glossary of Terms - 12/15 2 of 13 Acid Etching This process for the decoration of glass involves the application of hydrofluoric acid to the glass surface. Hydrofluoric acid vapors or baths of hydrofluoric acid salts may be used to give glass a matte, frosted appearance (similar to that obtained by surface sandblasting), as found in lighting glass. Glass designs can be produced by coating the glass with wax and then inscribing the desired pattern through the wax layer. When applied, the acid will corrode the glass but not attack the wax-covered areas. Alumina-silicate Glass Alumina (aluminum oxide Al2O3) is added to the glass batch in the form of commonly found feldspars containing alkalis in order to help improve chemical resistance and mechanical strength, and to increase viscosity at lower temperatures. Angle of Incidence The angle formed between a ray of light striking a surface and the normal line (the line perpendicular to the surface at that point). Annealing Under natural conditions, the surface of molten glass will cool more rapidly than the center. This results in internal stress- es which may cause the glass sheet or object to crack, shatter or even explode some time later. The annealing process is designed to eliminate or limit such stresses by submitting the glass to strictly controlled cooling in a special oven known as a “lehr”. Inside the lehr, the glass is allowed to cool to a temperature known as the “annealing point”. -

The Pennsylvania State University
The Pennsylvania State University The Graduate School ADVENTURES IN HIGH DIMENSIONS: UNDERSTANDING GLASS FOR THE 21ST CENTURY A Dissertation in Material Science and Engineering by Collin James Wilkinson © 2021 Collin James Wilkinson Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2021 ii The dissertation of Collin Wilkinson was reviewed and approved by the following: John Mauro Professor of Materials Science and Engineering Chair, Intercollege Graduate Degree Program Associate Head for Graduate Education, Materials Science and Engineering Dissertation Advisor Chair of Committee Seong Kim Professor of Chemical Engineering Professor of Materials Science and Engineering Ismaila Dabo Associate Professor of Materials Science and Engineering Susan Sinnott Professor of Materials Science and Engineering Professor of Chemistry Head of the Department of Materials Science and Engineering iii Abstract Glass is infinitely variable. This complexity stands as a promising technology for the 21st century since the need for environmentally friendly materials has reached a critical point due to climate change. However, such a wide range of variability makes new glass compositions difficult to design. The difficulty is only exaggerated when considering that not only is there an infinite variability in the compositional space, but also an infinite variability thermal history of a glass and in the crystallinity of glass-cearmics. This means that even for a simple binary glass there are at least 3 dimensions that have to be optimized. To resolve this difficulty, it is shown that energy landscapes can capture all three sets of complexity (composition, thermal history, and crystallinity). The explicit energy landscape optimization, however, has a large computational cost. -

Indoor Glass Cleaning Brochure
Clean Windows and Glass Faster, Safer and with Less Chemicals Indoor Glass Cleaning A Complete Line For All Glass Cleaning Needs ungerglobal.com Safely and Efficiently Clean All Your Indoor Glass Cleaning professionals face two key challenges when cleaning indoor surfaces such as windows, mirrors and elevators: safety and efficiency. Current window and glass cleaning tools lead to time-consuming and dangerous issues, like re-arranging furniture and reaching high or unusually positioned windows. Unger’s line of indoor window and glass cleaning tools increase productivity and safety by eliminating time spent moving furniture or climbing ladders. Clean Windows The Award Winning Choice 25% Faster and use for Indoor Glass Cleaning The Unger Stingray Ultimate Indoor 39% Less Chemicals Cleaning Tool provides a variety of glass and * window cleaning options, while the multiple than traditional spray and cloth cleaning lightweight extension poles enable you to clean high access areas without the use of ladders! The cleaning solution is powered by 3M Scotchgard™ Protection, which reduces the need to clean glass as often over time and can clean up to 1600 sq. ft. of windows before replacement. SRKT8 Stingray® Indoor Cleaning Kit - Deluxe 10’ shown *Source: Market Research – Professional Window Cleaners. Germany/UK, 2015, SR Strategy Routes Stingray® Indoor glass and window cleaning with Maximum Refillable System The Unger Stingray Flexibility, with new Refillable Refillable System allows ™ you to use your preferred Bottles and QuikPads window cleaning solution while still gaining all the QuikPad™ eliminates the need for laundering safety, speed and cleaning benefits Stingray provides. The QuikPad™ provides even more flexibility, allowing you to clean Adapter Plate used without having to launder with QuikPad™ Refillable bottle allows pads. -

Simple Chemical Experiments Simple Chemical Experiments
SIMPLE CHEMICAL EXPERIMENTS SIMPLE CHEMICAL EXPERIMENTS By ALFRED MORGAN Illustrated by THE AUTHOR APPLETON-CENTURY-CROFTS, INC. NEW YORK COPYRIGHT, 1941, BY D. APPLETON-CENTURY COMPANY, INC All rights reserved. This book, or parts thereof, must not be reproduced in any form without permission of the publisher. PRINTED IN THE UNITED STATES OF AMERICA CONTENTS CHAPTER PAGE I. YOUR LABORATORY i II. EXPERIMENTS WITH PRECIPITATES .... 26 III. EXPERIMENTS WITH SULFUR AND SOME OF ITS COMPOUNDS 54 IV. EXPERIMENTS WITH OXYGEN AND OXYGEN COM POUNDS 73 V. EXPERIMENTS WITH GASES AND SOME OF THEIR COMPOUNDS 103 VI. CHEMICAL TESTS 123 VII. SAFE "FIREWORKS" 144 VIII. EXPERIMENTS WITH A FEW ORGANIC COMPOUNDS 156 IX. CHEMICAL TRICKS AND MAGIC 170 X. MISCELLANEOUS EXPERIMENTS 186 XI. PRACTICAL USES FOR YOUR CHEMICAL KNOWL EDGE 214 XII. THE CHEMICALS YOU WILL NEED . .231 INDEX OF CHEMICALS 259 GENERAL INDEX 263 V SIMPLE CHEMICAL EXPERIMENTS *> CHAPTER I I YOUR LABORATORY I OST of the experiments described in this book can be M performed without elaborate equipment or apparatus. | For them you will need only a few bottles, test-tubes, meas- i uring-spoons, and an alcohol lamp. Jelly glasses, mayonnaise f jars, small enameled saucepans, and thin glass tumblers can | often be substituted for the beakers, flasks, and glassware of I the professional chemist. t A few of the experiments require beakers, flasks, tubing, | funnels, filter paper, crucibles, mortar and pestle, and Bunsen I burner. The small sizes of these are not expensive. Frequently the cost of apparatus and chemicals can be shared by estab lishing a "community" laboratory which is used by two or more experimenters. -

Bullseye Glass Catalog
CATALOG BULLSEYE GLASS For Art and Architecture IMPOSSIBLE THINGS The best distinction between art and craft • A quilt of color onto which children have that I’ve ever heard came from artist John “stitched” their stories of plants and Torreano at a panel discussion I attended a animals (page 5) few years ago: • A 500-year-old street in Spain that “Craft is what we know; art is what we don’t suddenly disappears and then reappears know. Craft is knowledge; art is mystery.” in a gallery in Portland, Oregon (page 10) (Or something like that—John was talking • The infinite stories of seamstresses faster than I could write). preserved in cast-glass ghosts (page 25) The craft of glass involves a lifetime of • A tapestry of crystalline glass particles learning, but the stories that arise from that floating in space, as ethereal as the craft are what propel us into the unknown. shadows it casts (page 28) At Bullseye, the unknown and oftentimes • A magic carpet of millions of particles of alchemical aspects of glass continually push crushed glass with the artists footprints us into new territory: to powders, to strikers, fired into eternity (page 31) to reactive glasses, to developing methods • A gravity-defying vortex of glass finding like the vitrigraph and flow techniques. its way across the Pacific Ocean to Similarly, we're drawn to artists who captivate Emerge jurors (and land on the tell their stories in glass based on their cover of this catalog) exceptional skills, but even more on their We hope this catalog does more than point boundless imaginations. -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position.