Equipment Detailsr07
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Era NMR Supplies and Accessories Catalog
analysco NMR Sample Tubes and Accessories from: Over the past twenty years, New Era has been providing the highest quality NMR sample tubes and accessories worldwide, keeping pace with new applications by offering alternative sampling techniques such as capillaries for metabolic samples and apparatus for RDC sample preparation and measurements. In addition, New Era has added a number of other new products to help make sample preparation and experiments easier and more efficient. You can look to New Era for innovation in sampling techniques. Analysco Ltd, 11 Woodlands Close, Milton under Wychwood, Chipping Norton, OX7 6LS, UK T / F: +44 (0)1993 832907 E: [email protected] W: www.analysco.co.uk CONTENTS Page No. Capillaries / Adapters / Support Rods. 21 Page No. Cleaning Brush for sample tubes . 25 NMR Sample Tubes, 3mm, 5mm . 4-7 Cleaning of sample tubes . 32 (including Quartz - 5mm) . 6 Coaxial Insert Cells. 19 NMR Sample Tubes, 8mm to 27mm . 8-9 Contact / Ordering Information . 2 (including Quartz - 10mm) . 8 Cross-Reference for Products. 29-31 NMR Tube Pressure / Volume Data . 32 NMR Tube Specifications . 32 Controlled Atmosphere Valve Sample Tubes . 17 Non-Glass Poly Sample Cells and Accessories Dewars for NMR Applications . 27 (Boron, Fluorine and Silicon studies) . 18 Distributors . 28 pH Electrodes and Solutions. 23 EPR (ESR) Sample Tubes (Quartz) . 19 Pipets / Rubber Bulbs . 22 Pressure Valve Sample Tubes . 17 Gel Sample Tubes (including Presses). 14-15 Probe Inserts (Quartz) . 26 Hazardous Sample Tube System . 16 Raman Sample Tubes . 19 Holders (Racks) for sample tubes . 22 Sample Tube Caps (including Teflon) . 20 Labels (Clear) for sample tubes . -

LAB: One Tube Reaction Part 1
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube, iron nail, sodium chloride, copper (II) sulfate, distilled water, glass stirring rod, tissue paper, parafilm wax Procedures: 1. Label a test tube to identify your group 2. Fill approx. 1/3 of the test tube with copper (II) sulfate crystals. Gently tap the tube to allow the crystals to settle. 3. Using a glass stirring rod, carefully cover the crystals with a layer of Kimwipe tissue paper. 4. Slowly, and with as little disturbance as possible, add enough distilled water to just cover the paper and blue crystals. 5. Repeat the process for the sodium chloride, filling approx. 1/3 of the test tube. Gently tap the tube to allow the crystals to settle. 6. Push more tissue paper into the test tube on top of the white crystals. 7. Add enough water to cover the tissue paper and white crystals. 8. Obtain an iron nail and expose the surface by rubbing with sand paper. 9. Carefully slide the nail into the test tube. 10. Continue adding water until the nail is completely covered. 11. Cover the test tube with parafilm wax and record your Day 1 observations. 12. Return the test tube to the test tube rack. 13. Continue to monitor and record your observations for the next several days, as indicated in the data table. Data: 1. Complete the NFPA label for the two chemicals used in this lab. -

Auxiliary Lab Manual Chem 465L Biochemistry II Lab Spring 2019
Auxiliary Lab Manual Chem 465L Biochemistry II Lab Spring 2019 1 Table of Contents Lab Safety 3 Report Sheet for Experiment 7a 8 A 31P NMR study of a Kinase Reaction 9 Overview of in Vitro and in Vivo 13C Yeast Experiments 12 In Vitro 13C Analysis of Yeast Metabolism 13 In Vivo 13C Analysis of Yeast Metabolism 18 Report Sheet for 13C Analysis of Yeast Metabolism 21 A Reminder of Kinetics Principles 22 NMR Analysis of the Fumarase Reaction 23 DNA Denaturation Lab I - UV 30 DNA Denaturation Lab II - IR 34 DNA Supercoiling Lab 41 DNA/RNA sequence analysis - Computer Lab 45 2 Principles for Safety in the Chemical Laboratory Safe practices in the chemical laboratory are of prime importance. A student should consider it an essential part of his or her educational experience to develop safe and efficient methods of operation in a lab. To do this, one must acquire a basic knowledge of properties of materials present in the lab, and one should realize the types of hazards that exist and the accidents and injuries that can result from ignorance or irresponsibility on the part of the student or a neighbor. Regulations 1. Wear safety goggles at all times while in the laboratory. 2. Report all accidents to the instructor or lab assistant immediately. 3. NEVER eat, drink, chew, or smoke in the laboratory. 4. NEVER leave an experiment unattended. Inform the lab assistant if you must leave the lab. 5. After the experiment is completed, turn all equipment off, making sure it is properly stored, and clean your area. -

NMR Facility User Guide
NMR Facility User Guide Analytical Instrumentation Center School of Pharmacy University of Wisconsin–Madison F2 (ppm) 1.5 2.0 2.5 3.0 3.5 4.0 4.5 75 70 65 60 55 50 45 40 35 30 F1 (ppm) Thomas C. Stringfellow Sixth Edition Version 2019-05-01 Table of Contents 1 Introduction 1 1.1 NMR Spectrometers .................................... 1 1.1.1 AV-400 Spectrometer ................................ 2 1.1.2 UI-500 Spectrometer ................................ 2 1.2 Supporting Computers and Software ............................ 5 1.3 NMR Facility Policies ................................... 6 1.3.1 Access and Use ................................... 6 1.3.2 User Training .................................... 6 1.3.3 Fees and Services .................................. 6 1.3.4 Reserving Instrument Time ............................. 6 1.3.5 Laboratory Safety and Health Issues ........................ 7 1.3.6 AV-400 Sample-Management Policies ....................... 9 1.3.7 Incident Report Form ................................ 10 2 Computers and Software 12 2.1 Information for Bruker Users ................................ 12 2.1.1 IconNMR Web View ................................ 13 2.2 Information for Varian Users ................................ 13 2.2.1 VNMR 6.1C Users Take Note! ........................... 13 2.2.2 Sun Desktop Environments: CDE versus OWD .................. 14 2.3 NMR Facility Computer Network ............................. 15 2.4 Samba Server Connections to NMR Facility Disk Shares ................. 15 2.4.1 Connection to Samba Shares from Microsoft Windows Clients .......... 16 2.4.2 Connection to Samba Shares from Mac OS X Clients ............... 17 2.5 X-Win32 Connectivity to Sun Computers ......................... 17 2.5.1 Installing X-Win32 ................................. 18 2.5.2 Configuring X-Win32 ............................... 19 2.5.3 Configuring the PC Monitor Resolution ..................... -

Documm Mgson!
Documm mgson! ED 174 412 SE 027 981 TITLE Biomedical Science, Unit I: Respiration inHealth and Medicine. Respiratory Anatomy, Physiology and Pathology; The Behavior of Gases;Introductory Chemistry; and Air Pollution. Laboratory Manual. Revised Version, 1975. INSTITUTION Biomedical Interdisciplinary Curriculum Project, Berkeley, Calif. SPONS AGENCY National Science Foundation, Washington, D.C. PUB DATE 75 NOTE 181p.; For related documents, see SE 027978-999 and SE 028 510-516; Not available in hard copydue to copyright restrictions EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIpTCRS Chemistry; Environment; Health; *HealthEducation; Higher Education; *Laboratory ExFeriments; *Laboratory Techniques; *Laboratory Training;Medical Education; Pathology; Physics; *Physiology;*Science Education; Secondary Education IDENTIFIERS *Respiration ABSTItACT Designed to accompany the student text on respiration, this manual presents instructions on the use of labcra tcry equipment and presents various experimentsdealing with the concepts presented in the text. Thirty=nine laboratoryactivities ' are de scribed. Laboratory activities are dividedinto several parts, each Part covering a specific experiment dealing with the concept covered by the activity. Each experimentincludes a description of materials, procedures, and discurision questions. (RE) ********************************************************************** Reproductions supplied by EDRS are the bestthat can be made from the original document. 4********************M414141#***********4141*****4141***********41#********4 -

Secure Series NMR Sample Tubes
in the past 50 years ...with help from Norell you have made new discoveries through cutting edge research creating a new world of possibilities with life- changing breakthroughs. Here’s to another 50 years with Norell by your side. Table of Contents Secure Series NMR Tubes Accessories Secure Series/NorLoc II 4 pH Electrodes & Cables for NMR Tubes 46 Secure 55 Series 5mm 5 Pasteur Pipettes 47 Secure 33 Series 5mm & 3mm 6, 7 Optimizer Inserts™ for 5mm Turbines 48, 49 Toroids 50 Select Series NMR Tubes Spinner Brushes 50 10mm Select Series 8 Fluoropolymer NMR Tube Liners 51 5mm Select Series 8 Fluoropolymer Liner Tube Kits 51 3mm & 4mm Select Series 9 3mm & 5mm NMR Tube Brush 51 5mm & 3mm NMR Sample Tubes for Ceramic Turbines 10, 11 72 Position NMR Tube Rack 52 5mm NMR Tube Carriers 52 Standard Series NMR Tubes Fluoropolymer NMR Tube Caps 52 5mm Standard Series 12, 13 NMR Tube Cleaner, 5 Position 53 10mm Standard Series 14, 15 NRS-250 Surfactant 53 Tube Washing Unit 53 Special Purpose Tubes NorLoc™ Generation II 5mm & 3mm Caps 4, 54, 55, 56, 57 Sample Vault™ NMR Tubes & Caps 16, 17, 18, 19 Economy NMR Tube Caps 58 Closures for Open Port Caps 18, 19 PTFE Syringe Tubing 59 Quartz NMR Tubes 20, 21 Cuvettes 60 Quartz EPR Tubes 21 Fluoropolymer Column Packing 60 Valved NMR Tubes 22, 23 Coaxial Inserts for NMR Tubes 61 Valved NMR Tubes for Intermediate Pressure 24, 25, 26, 27 Polypropylene Syringes 61 Valved NMR Tubes for High Pressure 28, 29, 30, 31 5mm & 10mm NMR Tube Septa 62 Screw-Cap NMR Tubes 32 Bruker Match™ NMR Tube Rack 62 Screw Cap NMR -

ACE GLASS INCORPORATED PRICE LIST 2021 Effective January 1, 2021
Custom Amber Coating ACE GLASS INCORPORATED PRICE LIST 2021 Effective January 1, 2021 Contact your local sales rep today for more information 1-800-223-4524 | www.aceglass.com Order by Code ACE Glassware Discounts Each item in this catalog has a two or three-digit code in addition to All ACE-manufactured glassware, identified with a spade (♠), listed the four or five-digit number. No other ordering information is needed in this catalog is subject to the following dollar value discounts. since each individual size, capacity, etc. has its own code. Example: Items marked by a star (★) or that have no designation whatsoever 5000 10/30 top 14/35 bottom would be ordered as 5000-05. are not subject to this discount. The majority of items listed in this catalog are normally available 10% on purchases of $500.00 and over from stock at our plant in Vineland, NJ. 12% on purchases of $1000.00 and over In the event your entire order cannot be filled immediately, a partial 15% on purchases of $1500.00 and over shipment will be sent, with the back-ordered items following shortly. Terms: Net 30 days (Domestic only) If you should desire the entire order to be sent in one shipment, Minimum Order: $25.00 please specify on your purchase order. Unless otherwise specified on the order, we will ship material by what we consider the “best way.” All quantities in this catalog are “each” unless otherwise noted. Ways to Order Contact us for current pricing or visit www.aceglass.com Ace Glass products are also available from our many lab distribution partners, particularly VWR International and Sigma Aldrich. -

Technique Notes
Dros. Inf. Serv. 90 (2007) 131 Technique Notes Methods and rationale for high-resolution magnetic resonance imaging (MRI) of Drosophila, using an 18.8 Tesla NMR spectrometer. Null, Brian1*, Corey W. Liu2, Maj Hedehus3, Steven Conolly4, and Ronald W. Davis1. 1Stanford Genome Technology Ctr/Bio-X Program; 2Stanford Magnetic Resonance Laboratory; 3Varian Inc, NMR Instruments; 4U.C. Berkeley, Dept. of Bioengineering; *Corresponding author Introduction Magnetic resonance imaging (MRI) is proven as an important tool for the study of thick or opaque tissues in living organisms, and its application to the study of development and biomedicine in the smallest model organisms is an exciting frontier. NMR spectrometers, while not typically used for imaging, are capable of generating extremely high magnetic fields, up to approximately 20 Tesla, whereas the more familiar MRI devices used to image humans in the clinical setting operate at only about one Tesla. High field strength is especially important for tiny specimens like the fruit fly, to increase signal to noise ratio and NMR spectral resolution for quantitation of metabolites in vivo. The small sample dimensions of the NMR spectrometer are ideal for the study of Drosophila and other small model organisms. Further, the ongoing innovation of MR contrast agents which can act as in vivo indicators of physiological status such as calcium ion concentration and gene expression, combined with the robustness of Drosophila as a model organism with a diverse array of genetic tools and genomic data, would make in vivo imaging and spectroscopy a highly desirable technique for the study of Drosophila. Over the past several decades, tremendous advances have been made in the capabilities of magnetic resonance methods for imaging and spectroscopic measurement in human subjects. -
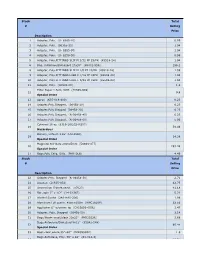
Stock Total # Selling Price 1 Adapter, Poly. (U- 6365-10)
Stock Total # Selling Price Description 1 Adapter, Poly. (U- 6365-10) 0.99 2 Adapter, Poly. (06365-22) 1.04 3 Adapter, Poly. (U- 6365-30) 1.04 4 Adapter, Poly. (U- 6359-50) 0.99 5 Adapter, Poly,FITTINGS SLIP M 3/32 PP 25/PK (45518-24) 1.04 6 Bag, Autoclave/Biohazard 24x30'' (95042-556) 156.1 8 Adapter, Poly.FITTINGS SLIP M 1/8 PP 25/PK (45518-26) 1.59 9 Adapter, Poly.FITTINGS LUER F 1/16 PP 25PK (45508-00) 1.04 10 Adapter, Poly.FITTINGS LUER F 3/32 PP 25PK (45508-02) 1.04 11 Adapter, Poly. (30800-08) 1.3 Filter Paper 1.5cm, VWR (28309-989) 12 9.9 Special Order 13 Apron (635-018-400) 0.23 14 Adapter,Poly.Stepped, (06458-10) 6.23 15 Adapter,Poly.Stepped (06458-20) 6.23 16 Adapter,Poly.Stepped, (N-06458-40) 6.23 17 Adapter,Poly.Stepped, (N-06458-60) 5.06 Cytoseal 16 oz (8310-16/23244257) 18 58.49 Hazardous Battery, Lithium 3.6V (LS14500) 19 14.38 Special Order Magnetic Stir Bars 2mmx5mm (58948-377) 20 293.48 Special Order 21 Bags,Poly Cello,10 lb. (PKR10Lb) 4.49 Stock Total # Selling Price Description 22 Adapter,Poly. Stepped (N-06458-30) 2.71 24 Alconox (21835-032) 42.75 25 Ammonium Sulfate,certif. (A7023) 41.18 26 Bar, spin 2'' x 1/2'' (14-51367) 5.22 27 Alcohol Swabs (248-HAS-200) 1.96 28 Aluminum Foil comm. 45cmx100m (WPC1810P) 32.56 29 Applicator 6'' w/cotton tip (CA10806-000L) 1.47 30 Adapter, Poly. -

Laboratory Equipment AP
\ \\ , f ?7-\ Watch glass 1 Crucible and cover Evaporating dish Pneumatlo trough Beaker Safety goggles Florence Wide-mouth0 Plastic wash Dropper Funnel flask collecting bottle pipet Edenmeyer Rubber stoppers bottle flask € ....... ">. ÿ ,, Glass rod with niohrome wire Scoopula (for flame re,sting) CruoiNe tongs Rubber ubing '1 ,v .... Test-tube brush square Wire gau ÿ "\ file Burner " Tripod Florence flask: glass; common sizes are 125 mL, 250 mL, 500 .d Beaker: glass or plastic; common sizes are 50 mL, mL; maybe heated; used in making and for storing solutions. 100 mL, 250 mL, 400 mL; glass beakers maybe heated. oÿ Buret: glass; common sizes are 25 mL and 50 mL; used to Forceps: metal; used to hold or pick up small objects. Funnel: glass or plastic; common size holds 12.5-cm diameter measure volumes of solutions in titrafions. Ceramic square: used under hot apparatus or glassware. filter paper. Gas burner: constructed of metal; connected to a gas supply Clamps" the following types of clamps may be fastened to with rubber tubing; used to heat chemicals (dry or in solution) support apparatus: buret/test-tube clamp, clamp holder, double buret clamp, ring clamp, 3-pronged jaw clamp. in beakers, test tubes, and crucibles. Gas collecting tube: glass; marked in mL intervals; used to 3: Clay triangle: wire frame with porcelain supports; used to o} support a crucible. measure gas volumes. Glass rod with nichrome wire: used in flame tests. Condenser: glass; used in distillation procedures. Q. Crucible and cover: porcelain; used to heat small amounts of Graduated cylinder: glass or plastic; common sizes are 10 mL, 50 mL, 100 mL; used to measure approximate volumes; must solid substances at high temperatures. -

Lab # 4: Separation of a Mixture
Name: Date Completed: Lab Partner(s): Lab # 4: Separation of a Mixture Lab Accelerated Chemistry 1 Objective You will be given a mixture containing sodium chloride (NaCl, table salt), benzoic acid (C7H6O2, a common food preservative), and silicon dioxide (SiO2, sand). Your goal is to separate the substances and determine the percent of each in the original mixture. Here is the separation scheme: Mixture (NaCl, benzoic acid, sand) Extract with 100°C H O 2 Decant residue supernatant Sand (wet) NaCl, benzoic acid Cool Filter evaporate H O 2 residue filtrate Sand (dry) Benzoic acid (wet) NaCl (solution) evaporate H2O Benzoic acid (dry) Introduction Mixtures are not unique to chemistry; we use and consume them on a daily basis. The beverages we drink each morning, the fuel we use in our automobiles, and the ground we walk on are mixtures. Very few materials we encounter are pure. Any material made up of two or more substances that are not chemically combined is a mixture. The isolation of pure components of a mixture requires the separation of one component from another. Chemists have developed techniques for doing this. These methods take advantage of the differences in physical properties of the components. The techniques to be demonstrated in this laboratory are the following: Extraction. This uses a solvent to selectively dissolve one component of the solid mixture. With this technique, a soluble solid can be separated from an insoluble solid. Decantation. This separates a liquid from insoluble solid sediment by carefully pouring the liquid from the solid without disturbing the solid. Recrystallization. -

Used for Moving Beakers Off of Hot Surfaces
Lab Equipment and Use Review Sheet Glassware Function Glassware Function Glassware Function Running Accurately Running reactions, measuring/ reactions, heating chemicals mixing mixing delivering chemicals – chemicals, volumes of easier for mixing Beaker heating Buret liquids than beakers chemicals Erlenmeyer Flask Used in Running Used to mix vacuum reactions, chemicals to heating filtration chemicals accurately mixing determine chemicals – concentration; Volumetric easier for contains exact Florence Flask Flask Filter Flask mixing than volumes beakers Used for Used for Used for mixing filtering and accurately and heating chemicals and for adding measuring running chemicals the volume reactions– without of liquids. smaller quantities Funnel Graduated spilling Test Tube than beakers and Cylinder flasks. Holding For stirring For storing Chemicals, Glass Stirring Rod chemicals small amounts covering of chemicals Watch Glass beakers during Sample Vial heating For adding Used to small evaporate amounts of Used for Evaporating liquids chemicals – lighting burner Dish usually by Plastic Pipets drops Used to add Utility Clamp – deionized used to hold water; to add objects on a solvents for ring stand. cleaning of Iron ring – Squirt Bottle beakers and used to hold other objects above glassware. a Bunsen burner flame. Ring Stand Ring stand – Beaker Tongs with Utility used to hold Clamp and various Iron Ring objects. Equipment Function Equipment Function Equipment Function Heat source in Used to grind Used to clean the chemistry chemicals into glassware lab. Uses powder natural gas. Mortar and Pestle Test tube brush/beaker Bunsen Burner brush Used to hold a Used to Used to crucible above transport hot strongly heat a flame – used Crucible tongs crucibles and substances Clay triangle in conjunction to remove Crucible and above a flame with iron ring their covers Cover Used to hold Used to handle a Used in group of test single test tube.