Chemistry Lab Safety Quiz Example
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LAB: One Tube Reaction Part 1
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube, iron nail, sodium chloride, copper (II) sulfate, distilled water, glass stirring rod, tissue paper, parafilm wax Procedures: 1. Label a test tube to identify your group 2. Fill approx. 1/3 of the test tube with copper (II) sulfate crystals. Gently tap the tube to allow the crystals to settle. 3. Using a glass stirring rod, carefully cover the crystals with a layer of Kimwipe tissue paper. 4. Slowly, and with as little disturbance as possible, add enough distilled water to just cover the paper and blue crystals. 5. Repeat the process for the sodium chloride, filling approx. 1/3 of the test tube. Gently tap the tube to allow the crystals to settle. 6. Push more tissue paper into the test tube on top of the white crystals. 7. Add enough water to cover the tissue paper and white crystals. 8. Obtain an iron nail and expose the surface by rubbing with sand paper. 9. Carefully slide the nail into the test tube. 10. Continue adding water until the nail is completely covered. 11. Cover the test tube with parafilm wax and record your Day 1 observations. 12. Return the test tube to the test tube rack. 13. Continue to monitor and record your observations for the next several days, as indicated in the data table. Data: 1. Complete the NFPA label for the two chemicals used in this lab. -

Safety in the Chemistry Lab
Safety in the Chemistry Lab Working in the chemistry laboratory is an inter- 12. Never touch any substance in the lab un- esting and rewarding experience. During your less specifically instructed to do so by your labs, you will be actively involved from beginning teacher. to end—from setting some change in motion to 13. Never put your face near the mouth of a drawing some conclusion. In the laboratory, you container that is holding chemicals. will be working with equipment and materials that 14. Never smell any chemicals unless in- can cause injury if they are not handled properly. structed to do so by your teacher. When testing for However, the laboratory is a safe place to work if odors, use a wafting motion to direct the odors to you are careful. Accidents do not just happen, they your nose. are caused—by carelessness, haste, and disregard 15. Any activity involving poisonous vapors of safety rules and practices. Safety rules to be should be conducted in the fume hood. followed in the laboratory are listed below. Before 16. Dispose of waste materials as instructed beginning any lab work, read these rules, learn by your teacher. them, and follow them carefully. 17. Clean up all spills immediately. 18. Clean and wipe dry all work surfaces at General the end of class. Wash your hands thoroughly. 19. Know the location of emergency equip- 1. Be prepared to work when you arrive at ment (first aid kit, fire extinguisher, fire shower, the laboratory. Familiarize yourself with the lab fire blanket, etc.) and how to use them. -

Equipment Detailsr07
Lab Equipment Details Lab Equipment Glass Flasks 150ml 250ml 500ml Lab Equipment Glass Beakers 150ml 250ml 500ml Lab Equipment Glassware But once removed, only the cap stays highlighted. Droppers critical to Lab An activated Dropper coursework can be found highlights the entire bottle. already in the workspace. Dropper Dropper Dropper Activated In Use Lab Equipment Gastight Syringe Small A pre-filled gastight syringe can be used with a NMR tube to safely fill through the top in preparation for use with the NMR spectrometer. Gastight Syringe NMR Tube with Holder with Holder Lab Equipment NMR Tube Spinner An NMR Tube filled with gas for use with the NMR Spectrometer Simply use the NMR Tube with needs to be inserted into the the Tube Spinner. Spinner before it can be used. NMR Tube NMR Tube Spinner NMR Tube Spinner with Holder with NMR Tube inserted Once the Tube slotted into the holder is inserted into the top part of the spectrometer, users can type in a number for Lab Equipment the frequency they’d like to scan. NMR Spectrometer XL The NMR Tube holder can then be slotted into the highlighted tube on the NMR Spectrometer. NMR Tube filled with appropriate substance is slotted (used with) in the holder. Lift will perpare the Spectrometer for the tube holder insertion. Scan No. Allows the user to change the frequency at which the tube is scanned The NMR Tube holder can then be slotted into the top tube on Lab Equipment the NMR Spectrometer. NMR Spectrometer XL NMR Tube filled with appropriate substance is slotted (used with) in the holder. -

Whoosh Bottle
Whoosh Bottle Introduction SCIENTIFIC Wow your students with a whoosh! Students will love to see the blue alcohol flame shoot out the mouth of the bottle and watch the dancing flames pulsate in the jug as more air is drawn in. Concepts • Exothermic reactions • Activation energy • Combustion Background Low-boiling alcohols vaporize readily, and when alcohol is placed in a 5-gallon, small-mouthed jug, it forms a volatile mixture with the air. A simple match held by the mouth of the jug provides the activation energy needed for the combustion of the alcohol/air mixture. Only a small amount of alcohol is used and it quickly vaporizes to a heavier-than-air vapor. The alcohol vapor and air are all that remain in the bottle. Alcohol molecules in the vapor phase are farther apart than in the liquid phase and present far more surface area for reaction; therefore the combustion reaction that occurs is very fast. Since the burning is so rapid and occurs in the confined space of a 5-gallon jug with a small neck, the sound produced is very interesting, sounding like a “whoosh.” The equation for the combustion reaction of isopropyl alcohol is as follows, where 1 mole of isopropyl alcohol combines with 4.5 moles of oxygen to produce 3 moles of carbon dioxide and 4 moles of water: 9 (CH3)2CHOH(g) + ⁄2O2(g) → 3CO2(g) + 4H2O(g) ∆H = –1886.6 kJ/mol Materials Isopropyl alcohol, (CH3)2CHOH, 20–30 mL Graduated cylinder, 25-mL Whoosh bottle, plastic jug, 5-gallon Match or wood splint taped to meter stick Fire blanket (highly recommended) Safety shield (highly recommended) Funnel, small Safety Precautions Please read all safety precautions before proceeding with this demonstration. -

Fire Prevention Program Revised September 2018 Page 1 of 10 Obey All “No Smoking” and “No Open Flame” Signs and Postings
FIRE PREVENTION & PROTECTION PROGRAM PURPOSE / SCOPE The purpose or goal of the Fire Extinguisher Program is to inform and train employees on preventing fires in the workplace and the proper selection and the use of fire extinguishers in the event of an incipient fire. GENERAL REQUIREMENTS Fire is a common and serious hazard in the construction industry. Each year fires take many lives, cause workers and their families to suffer, and cost millions of dollars. Fire prevention is everyone’s responsibility. Employees must do their part by observing and complying with fire-prevention regulations and procedures. Employees should report any potential fire hazard or condition that could cause a fire to their supervisor immediately. Many potential fire hazards that are commonly found on job sites include gasoline, diesel fuel, oxygen, acetylene and various other building materials. Most fires are caused by a violation of basic fire safety precautions. While proper procedures and training can minimize the chances of an accidental fire, employees must still be prepared to deal with a fire emergency should it occur. In accordance with OSHA standards, our employees must adhere to the following written Fire Prevention and Protection Program. A copy of this program shall be kept at the work place and is available for employee review. Fire extinguishers shall be readily available and located so that personnel do not have to travel more than 75 feet to reach one. On job sites, all Winger Companies, herein referred to as Winger, fire extinguishers SHALL be conspicuously located, readily accessible, and immediately available in the event of a fire for all cutting, welding and grinding operations. -

Flinn Scientific 2019 Purchase Guide a Quick and Easy Checklist of Science Essentials
Flinn Scientific 2019 Purchase Guide A Quick and Easy Checklist of Science Essentials Use this Purchase Guide as a handy tool for: • Taking Inventory • Order Preparation • Budget Management • Future Planning See your Flinn Scientific Catalog/Reference Manual SCIENTIFIC or visit www.flinnsci.com for product details. It’s Easy to Order Tom Trapp from Flinn Scientific! National Account Development Consultant [email protected] www.flinnsci.com/tom-trapp/sa1001 Online 402-960-5578 (mobile) www.flinnsci.com Offering personal assistance to help meet your science curriculum, supply, and lab safety needs. Email [email protected] Quality Products, Fast Delivery, Fax and Low Prices Guaranteed 1-866-452-1436 (toll free) Mail Flinn Scientific, Inc. P.O. Box 219 Batavia, IL 60510-0219 Phone 1-800-452-1261 7:30 am to 5:00 pm CT Monday through Friday Our Guarantee Flinn Scientific, Inc. guarantees that no sale is complete unless the customer is satisfied. Every item we furnish will either conform to the catalog specification, or we will ask your permission, prior to shipment, to ship an alternative product. If you find a lower published nationally advertised catalog price for an identical item, Flinn will “meet or beat” that price. Use this purchase guide containing popular product recommendations ©2019 Flinn Scientific, Inc. All Rights Reserved. to prepare your order, take inventory, and manage your budget. 1 www.flinnsci.com Flinn Scientific 2019 Purchase Guide 1 Item Rec. Item Rec. Product / Item Name Qty 2019 Price Total Product / Item Name Qty 2019 Price Total No. Qty No. Qty Safety & Personal Protection Equipment Aspirator, Water, Polypropylene AP1203 1 $ 19.30 $ - Apron, rubberized, 27" W X 36" L AP7125 30 $ 15.00 $ - Autoclave, Electric, Portable AP1004 1 $ 865.20 $ - Apron, plastic, 30" W x 36" L AP7120 30 $ 7.25 $ - ♦ Balance, Flinn Triple Beam OB2181 $ 115.00 $ - Gloves, Butyl rubber for conc. -

Specific Lab Safety Procedure Johnson's Group
Specific Lab Safety Procedure Johnson’s Group Last Edited: 1/18/2018 1. Purpose The purpose of this procedure is to support work practices for protecting laboratory personnel from potential health hazards in the laboratory. 1. Laboratory Safety Guidelines 1.1 GENERAL LABORATORY SAFETY • Do not eat, drink, or apply cosmetics in the lab. • Store food and drink in food designated refrigerators only. Don’t mix chemicals and food. • Tie back medium length and long hair and remove or secure dangling pieces of clothing (e.g. ties, draw-strings, headphones, etc. ) when working near flames or entangling equipment. • All accidents, no matter how minor, should be reported to the faculty/staff member supervising the laboratory. • Know the location of all safety equipment (e.g. eyewash, fire extinguisher, fire blanket, safety showers, spill kit) if available. • Keep aisles clear. • Maintain unobstructed access to all exits, fire extinguishers, electrical panels, emergency showers, and eyewashes. • Do not use corridors for storage or work areas. • Do not store heavy items above 6 feet high. • Consult with your Principal Investigator if planning to work alone or running an unattended operation. • Avoid working alone in the lab when performing high-risk operations. • Keep area clean and uncluttered; clean up area upon completion of task or at end of the day. 1.2 PERSONAL PROTECTIVE EQUIPMENT (PPE) • Review SOP, MSDS and other hazard information to determine appropriate PPE to wear based on chemical hazards encountered. • Remove gloves when leaving the laboratory, so as not to contaminate doorknobs, etc. 1.3 ELECTRICAL SAFETY • Don’t use permanent extension cords. -

Documm Mgson!
Documm mgson! ED 174 412 SE 027 981 TITLE Biomedical Science, Unit I: Respiration inHealth and Medicine. Respiratory Anatomy, Physiology and Pathology; The Behavior of Gases;Introductory Chemistry; and Air Pollution. Laboratory Manual. Revised Version, 1975. INSTITUTION Biomedical Interdisciplinary Curriculum Project, Berkeley, Calif. SPONS AGENCY National Science Foundation, Washington, D.C. PUB DATE 75 NOTE 181p.; For related documents, see SE 027978-999 and SE 028 510-516; Not available in hard copydue to copyright restrictions EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIpTCRS Chemistry; Environment; Health; *HealthEducation; Higher Education; *Laboratory ExFeriments; *Laboratory Techniques; *Laboratory Training;Medical Education; Pathology; Physics; *Physiology;*Science Education; Secondary Education IDENTIFIERS *Respiration ABSTItACT Designed to accompany the student text on respiration, this manual presents instructions on the use of labcra tcry equipment and presents various experimentsdealing with the concepts presented in the text. Thirty=nine laboratoryactivities ' are de scribed. Laboratory activities are dividedinto several parts, each Part covering a specific experiment dealing with the concept covered by the activity. Each experimentincludes a description of materials, procedures, and discurision questions. (RE) ********************************************************************** Reproductions supplied by EDRS are the bestthat can be made from the original document. 4********************M414141#***********4141*****4141***********41#********4 -

3.9 Fire Safety Equipment Fire Extinguishers Are Very Important
3.9 Fire Safety Equipment 3.9.1 Fire Extinguishers Fire extinguishers are very important components of laboratory safety. Fire extinguishers are spaced and located as required by current fire codes and standards. Currently the department of Environmental Health, Safety and Risk Management performs monthly inspection and performs required maintenance on all fire extinguishers at Stephen F. Austin State University. Only use a fire extinguisher if the fire is very small and you know how to use the extinguisher safely. If you can’t put out the fire, leave immediately. Make sure you call 911 or fire department even if you think the fire is out. In laboratories, fire extinguishers should be securely located on the wall near an exit. The lab occupant should be aware of the condition of the fire extinguishers by observing them for broken seals, damages, low gauge pressure, or improper mounting. Occupants of laboratories should visually inspect lab fire extinguishers at least monthly. Units that are missing, have broken seals, low pressure or visible damage should be reported to EHSRM immediately for replacement. For fire extinguisher service, requests, training, or any questions call EHSRM at 468- 4532. 3.9.2 Fire Alarms Fire alarms are designed so that all endangered laboratory personnel and building occupants are alerted by an audible warning (in many buildings there is also visual warning). All employees/students should become familiar with the exact location of the fire alarm pull stations nearest to their laboratory. Sprinkler systems, smoke detectors and heat detectors may automatically activate the fire alarm. (This should not be considered a substitute for manual fire alarm activation.) Smoke detectors should never be tampered with and must be regularly checked for viable battery. -
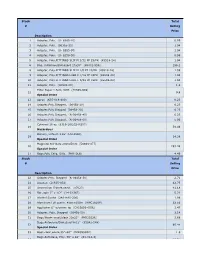
Stock Total # Selling Price 1 Adapter, Poly. (U- 6365-10)
Stock Total # Selling Price Description 1 Adapter, Poly. (U- 6365-10) 0.99 2 Adapter, Poly. (06365-22) 1.04 3 Adapter, Poly. (U- 6365-30) 1.04 4 Adapter, Poly. (U- 6359-50) 0.99 5 Adapter, Poly,FITTINGS SLIP M 3/32 PP 25/PK (45518-24) 1.04 6 Bag, Autoclave/Biohazard 24x30'' (95042-556) 156.1 8 Adapter, Poly.FITTINGS SLIP M 1/8 PP 25/PK (45518-26) 1.59 9 Adapter, Poly.FITTINGS LUER F 1/16 PP 25PK (45508-00) 1.04 10 Adapter, Poly.FITTINGS LUER F 3/32 PP 25PK (45508-02) 1.04 11 Adapter, Poly. (30800-08) 1.3 Filter Paper 1.5cm, VWR (28309-989) 12 9.9 Special Order 13 Apron (635-018-400) 0.23 14 Adapter,Poly.Stepped, (06458-10) 6.23 15 Adapter,Poly.Stepped (06458-20) 6.23 16 Adapter,Poly.Stepped, (N-06458-40) 6.23 17 Adapter,Poly.Stepped, (N-06458-60) 5.06 Cytoseal 16 oz (8310-16/23244257) 18 58.49 Hazardous Battery, Lithium 3.6V (LS14500) 19 14.38 Special Order Magnetic Stir Bars 2mmx5mm (58948-377) 20 293.48 Special Order 21 Bags,Poly Cello,10 lb. (PKR10Lb) 4.49 Stock Total # Selling Price Description 22 Adapter,Poly. Stepped (N-06458-30) 2.71 24 Alconox (21835-032) 42.75 25 Ammonium Sulfate,certif. (A7023) 41.18 26 Bar, spin 2'' x 1/2'' (14-51367) 5.22 27 Alcohol Swabs (248-HAS-200) 1.96 28 Aluminum Foil comm. 45cmx100m (WPC1810P) 32.56 29 Applicator 6'' w/cotton tip (CA10806-000L) 1.47 30 Adapter, Poly. -

Paper Mills: Guidance on Fire Risk 2 of 41 Pages Health and Safety Executive
Health and Safety Executive Paper mills Guidance on fire risk Paper and Board Industry Advisory Committee Health and Safety Executive This is a revision of guidance originally published in 1995. It has been prepared, in consultation with HSE, by the Paper and Board Industry Advisory Committee, which was appointed by the Health and Safety Commission as part of its formal advisory structures. The guidance represents what is considered to be good practice by the members of the Committee. It was agreed by the Commission. Following this guidance is not compulsory and you are free to take other action. But if you do follow this guidance you will normally be doing enough to comply with the law. Health and safety inspectors seek to secure compliance with the law and may refer to this guidance as illustrating good practice. The Regulatory Reform (Fire Safety Order) 2005 During the life of this document, the Regulatory Reform (Fire Safety) Order 2005 will come into force. This will repeal the Fire Precautions Act 1971 and other related legislation under which fire certificates are currently issued. It will also repeal the fire certificates themselves. At this point, general fire precautions then become the responsibility of the duty holder, who has to provide a risk assessment. This determines the general fire precautions needed and fire brigades will continue to enforce general fire precautions under the new legislation. This does not affect the advice given in the following pages except that the reader should bear in mind that the requirement for a paper mill to have a fire certificate prepared by the Fire Authority (mentioned in paragraphs 23, 55, 108, 112, 126 and 134) will be replaced by a specific requirement for the company to perform a risk assessment for fire safety. -

NEW PRODUCTS NOW AVAILABLE Fire Safety and Signage Products Issue 01 WELCOME
NEW PRODUCTS NOW AVAILABLE Fire Safety and Signage Products Issue 01 WELCOME Established in 1978 Jactone Products Ltd is a privately owned UK manufacturer specialising in the production and supply of a unique range of fire, safety and signage products. Our policy of continuous investment in technology and product development allows us to offer our unique brand of quality products at exceptional value. We are certified by BSI to BSI EN ISO 9001, covering all of our manufacturing operations and carry many third party approvals across our range of products. We have chosen to use BSI and LPCB approval schemes to ensure our customers have complete peace of mind. MANUFACTURER INNOVATION TECHNICAL EXPERTISE EFFICIENCY QUALITY FIRE EXTINGUISHERS MADE IN THE UK FULLY CERTIFIED Jactone Premium Range fire extinguishers The Premium Range extinguishers are are manufactured in our UK purpose built manufactured under our BS EN ISO 9001 factory in the heart of the West Midlands. quality system and they are fully certified Our advanced deep draw cylinder with third party approvals. technology produces a unique, quality fire extinguisher with a robust safety record. BRANDING A fantastic way to present and promote the corporate image of your organisation. COLOUR CODED Utilising our state of the art digital print and graphics suite we can take your Manufactured with a unique company logo and brand and create colour coding system in bespoke extinguisher header labels that accordance with the guidelines in will showcase you. BS 7863:2009. In the event of a fire, your staff will easily and quickly be able to identify the extinguisher required.