Suxamethonium Chloride 50 Mg/Ml Solution for Injection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Galantamine Potentiates the Neuroprotective Effect of Memantine Against NMDA-Induced Excitotoxicity Joao~ P
Galantamine potentiates the neuroprotective effect of memantine against NMDA-induced excitotoxicity Joao~ P. Lopes1, Glauco Tarozzo1, Angelo Reggiani1, Daniele Piomelli1,2 & Andrea Cavalli1,3 1D3 – Drug Discovery and Development Department, Istituto Italiano di Tecnologia, Via Morego, 16163, Genova, Italy 2Departments of Anatomy and Neurobiology and Biological Chemistry, University of California, Irvine, CA, 92697-4621 3Department of Pharmacy and Biotechnologies, Alma Mater Studiorum, Bologna University, Via Belmeloro, 40126, Bologna, Italy Keywords Abstract Alzheimer’s disease, drug combination, N NMDA neurotoxicity, NR2B, The combination of memantine, an -methyl-D-aspartate (NMDA) receptor polypharmacology, primary cortical neurons antagonist, with an acetylcholinesterase inhibitor (AChEI) is the current stan- dard of care in Alzheimer’s disease (AD). Galantamine, an AChEI currently Correspondence marketed for the treatment of AD, exerts memory-enhancing and neuroprotec- Andrea Cavalli, D3 – Drug Discovery and tive effects via activation of nicotinic acetylcholine receptors (nAChRs). Here, Development Department, Istituto Italiano we investigated the neuroprotective properties of galantamine in primary cul- di Tecnologia – Via Morego, 30, 16163 tures of rat cortical neurons when given alone or in combination with meman- Genova, Italy. Tel: +39 010 71781530; Fax: +39 010 tine. In agreement with previous findings, we found that memantine was fully 71781228; E-mail: [email protected] effective in reversing NMDA toxicity at concentrations of 2.5 and 5 lmol/L. Galantamine also completely reversed NMDA toxicity at a concentration of Funding Information 5 lmol/L. The a7 and a4b2 nAChR antagonists, methyllycaconitine, and dihy- No funding information provided. dro-b-erythroidine blocked the neuroprotective effect of galantamine, demon- strating the involvement of nAChRs. -

Comparative Study of Different Doses of Rocuronium Bromide For
MedDocs Publishers Annals of Anesthesia and Pain Medicine Open Access | Research Article Comparative study of different doses of rocuronium bromide for endotracheal intubation Nidhi V Sardhara1*; Sonal A Shah2; Dhaval P Pipaliya3; Shivansh Gupta3 1SVP hospital, 13th floor, Room no 13125, Near Ellis bridge, Ahmedabad, Gujarat-380006 2Assistant Professor, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India 3First year Resident, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India *Corresponding Author(s): Nidhi V Sardhara Abstract SVP hospital, 13th floor, Room no 13125, Near Ellis Background: Endotracheal intubation is one of such bridge, Ahmedabad, Gujarat-380006 development without which general anesthesia cannot be Tel: +91-9428-44-7878, Fax: 7926-57-8452 considered safe for any major surgery particularly head and Email: [email protected] neck, thoracic and abdominal surgeries. Ever since the ad- vent of anesthesia, anesthesiologists have been in search of an ideal muscle relaxants which can provide ideal intu- bating conditions in ultrashort duration with minimal side effects. Rocuronium bromide provides fast onset of action, Received: Mar 11, 2020 an intermediate duration of action and rapid recovery, good Accepted: Apr 24, 2020 to excellent intubating conditions at doses having minimal Published Online: Apr 30, 2020 or no haemodynamic changes. Present study is to compare the effect of different doses (0.6 mg/kg, 0.9 mg/kg, 1.2 mg/ Journal: Annals of Anesthesia and Pain Medicine kg) of Rocuronium bromide for endotracheal intubation at Publisher: MedDocs Publishers LLC 60 seconds. Online edition: http://meddocsonline.org/ Material and methods: This study was carried out by Copyright: © Sardhara NV (2020). -

Reference List of Drugs with Potential Anticholinergic Effects 1, 2, 3, 4, 5
ANTICHOLINERGICS: Reference List of Drugs with Potential Anticholinergic Effects 1, 2, 3, 4, 5 J Bareham BSP © www.RxFiles.ca Aug 2021 WHENEVER POSSIBLE, AVOID DRUGS WITH MODERATE TO HIGH ANTICHOLINERGIC ACTIVITY IN OLDER ADULTS (>65 YEARS OF AGE) Low Anticholinergic Activity; Moderate/High Anticholinergic Activity -B in combo Beers Antibiotics Antiparkinsonian Cardiovascular Agents Immunosuppressants ampicillin *ALL AVAILABLE AS amantadine SYMMETREL atenolol TENORMIN azaTHIOprine IMURAN cefOXitin GENERIC benztropine mesylate COGENTIN captopril CAPOTEN cyclosporine NEORAL clindamycin bromocriptine PARLODEL chlorthalidone GENERIC ONLY hydrocortisone CORTEF gentamicin (Oint & Sol’n NIHB covered) carbidopa/levodopa SINEMET digoxin LANOXIN, TOLOXIN methylprednisolone MEDROL piperacillin entacapone COMTAN dilTIAZem CARDIZEM, TIAZAC prednisone WINPRED dipyridamole PERSANTINE, ethopropazine PARSITAN vancomycin phenelzine NARDIL AGGRENOX disopyramide RYTHMODAN Muscle Relaxants pramipexole MIRAPEX Antidepressants baclofen LIORESAL ( on intrathecal only) procyclidine KEMADRIN furosemide LASIX amitriptyline ELAVIL cyclobenzaprine FLEXERIL selegiline ELDEPRYL hydrALAZINE APRESOLINE clomiPRAMINE ANAFRANIL isosorbide ISORDIL methocarbamol ROBAXIN OTC trihexyphenidyl ARTANE desipramine NORPRAMIN metoprolol LOPRESOR orphenadrine NORFLEX OTC doxepin >6mg SINEQUAN Antipsychotics NIFEdipine ADALAT tiZANidine ZANAFLEX A imipramine TOFRANIL quiNIDine GENERIC ONLY C ARIPiprazole ABILIFY & MAINTENA -
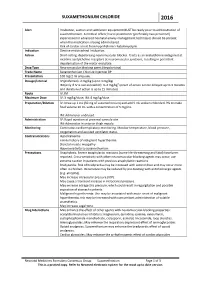
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

Case Report Carbamazepine Toxicity Following Oxybutynin And
Spinal Cord (2005) 43, 252–255 & 2005 International Spinal Cord Society All rights reserved 1362-4393/05 $30.00 www.nature.com/sc Case Report Carbamazepine toxicity following Oxybutynin and Dantrolene administration: a case report T Vander*,1, H Odi1, V Bluvstein1, J Ronen1,2 and A Catz1,2 1Department of Spinal Rehabilitation, Loewenstein Rehabilitation Hospital, Raanana, Israel; 2Tel-Aviv University, Tel-Aviv, Israel Objective: To report a case of Carbamazepine toxicity following the administration of Oxybutynin and Dantrolene. Study design: A case report. Setting: The Spinal Rehabilitation Department, Loewenstein Hospital, Raanana, Israel. Methods: A patient with C6D tetraplegia who sustained intoxication because of drug interaction is presented. She had been treated by Carbamazepine 1000 mg/day for neuropathic pain for 2 years without clinical or laboratory signs of toxicity. After administration of Oxybutynin concomitantly with an increase in the dose of Dantrolene, she presented the clinical symptoms and laboratory finding of Carbamazepine intoxication. Trying to adjust the treatment to the patient’s requirements, Carbamazepine together with Oxybutynin and Dantrolene was readministrated in lower doses. Results: The combination of these drugs, even small doses, caused toxicity. Adding Dantrolene and Oxybutynin elevated the blood level of Carbamazepine, possibly by inhibition of cytochrome P450. Conclusion: A possible pharmacokinetic interaction between Dantrolene and Oxybutynin should be borne in mind when considering Carbamazepine medication for a patient with a spinal cord lesion. Spinal Cord (2005) 43, 252–255. doi:10.1038/sj.sc.3101689; Published online 1 February 2005 Keywords: Carbamazepine; Oxybutynin; Dantrolene; drug interaction; cytochrome P450 Introduction Both Oxybutynin chloride and Dantrolene sodium are be required when patients have concomitant post- widely used in patients with spinal cord lesion (SCL). -

Structural and Functional Neuroprotection in Glaucoma: Role of Galantamine-Mediated Activation of Muscarinic Acetylcholine Receptors
Citation: Cell Death and Disease (2010) 1, e27; doi:10.1038/cddis.2009.23 & 2010 Macmillan Publishers Limited All rights reserved 2041-4889/10 www.nature.com/cddis Structural and functional neuroprotection in glaucoma: role of galantamine-mediated activation of muscarinic acetylcholine receptors M Almasieh1, Y Zhou1, ME Kelly2, C Casanova3 and A Di Polo*,1 Glaucoma is the leading cause of irreversible blindness worldwide. Loss of vision due to glaucoma is caused by the selective death of retinal ganglion cells (RGCs). Treatments for glaucoma, limited to drugs or surgery to lower intraocular pressure (IOP), are insufficient. Therefore, a pressing medical need exists for more effective therapies to prevent vision loss in glaucoma patients. In this in vivo study, we demonstrate that systemic administration of galantamine, an acetylcholinesterase inhibitor, promotes protection of RGC soma and axons in a rat glaucoma model. Functional deficits caused by high IOP, assessed by recording visual evoked potentials from the superior colliculus, were improved by galantamine. These effects were not related to a reduction in IOP because galantamine did not change the pressure in glaucomatous eyes and it promoted neuronal survival after optic nerve axotomy, a pressure-independent model of RGC death. Importantly, we demonstrate that galantamine- induced ganglion cell survival occurred by activation of types M1 and M4 muscarinic acetylcholine receptors, while nicotinic receptors were not involved. These data provide the first evidence of the clinical potential -

Skeletal Muscle Relaxants Review 12/17/2008
Skeletal Muscle Relaxants Review 12/17/2008 Copyright © 2007 - 2008 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 5181 Natorp Blvd., Suite 205 Mason, Ohio 45040 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Drug Class Review on Skeletal Muscle Relaxants
Drug Class Review on Skeletal Muscle Relaxants Final Report Update 2 May 2005 Original Report Date: April 2003 Update 1 Report Date: January 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Roger Chou, MD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2005 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS Introduction........................................................................................................................4 Scope and -

Zambia Essential Medicine List (Zeml) 03 2013
ZAMBIA ESSENTIAL MEDICINE LIST (ZEML) 03 2013 Drug Presentation Level VEN 1 Drugs used in anaesthesia 1.1. Drugs used in general anaesthesia 1.1.1 Intravenous and intramuscular anaesthetics 1.1.1.1 Ketamin injection 10mg/ml (10 ml) II-IV V 1.1.1.2 Thiopentone sodium powder for reconstitution 1g and 5g vials II-IV V 1.1.2 Inhalation anaesthetics 1.1.2.1 Halothane inhalation II-IV V 1.1.2.2 Nitrous oxide medical gas II-IV E 1.1.3 Muscle relaxants 1.1.3.1 Suxamethonium chloride injection 50mg/ml, (2ml) II-IV V 1.1.4 Anticholinesterases 1.1.4.1 Neostigmine injection 2.5mg/ml, (1ml) II-IV V 1.2 Drugs used in local anaesthesia 1.2.1. Lignocaine injection 1% (10ml, 50ml) I-IV V 1.2.2 Lignocaine + adrenaline dental cartridge injection 2% (1 in 80,000) II-IV V 1.3 Drugs used in spinal anaesthesia 1.3.1 Bupivacaine/glucose injection 0.5%, (4ml) IV E 1 Drug Presentation Level VEN 2. Drugs acting on the gastrointestinal system 2.1 Antacids 2.1.1 Aluminium hydroxide gel, chewable tablets I-IV E 2.12 Magnesium trisilicate Compound chewable tablets, mixture I-IV E 2.2. Antispasmodics 2.2.1 Hyoscine butyl bromide injection 20mg/ml, (1ml) II-IV E 2.2.2 Propantheline bromide tablets 15mg I-IV E 2.3 Ulcer healing drugs 2.3.1 Cimetidine tablets 200mg II-IV E 2.3.2 Omeprazole tablets 10mg, II-IV E 2.3.3 Ranitidine tablets 150mg II-IV E 2.3.4 Tripotassium dicitratobismuthate tablets 120mg II-IV E 2.3.5 Clarithromycin tablets 250mg II-IV E 2.4. -

2018 Product List Expertise in Injectables
2018 Product list Expertise in injectables Flucloxacillin ................................................................................ ................................................... 250 mg Antibiotics ................................................................................ ................................................... Flucloxacillin 500 mg Amoxicillin ....................................................................................... .................................................... 250 mg ................................................................................ .................................................................. Flucloxacillin 1 g Amoxicillin ....................................................................................... .................................................... 500 mg ..................................................................................... .................................................................. Fosfomycin 1 g Amoxicillin ....................................................................................... ................................................................... 1 g ..................................................................................... .................................................................. Fosfomycin 4 g Amoxicillin ....................................................................................... ................................................................... 2 g Gentamicin ...................................................................................... -

Potencies and Selectivities of Inhibitors of Acetylcholinesterase and Its Molecular Forms in Normal and Alzheimer’S Disease Brain*
Acta Biologica Hungarica 54 (2), pp. 183–189 (2003) POTENCIES AND SELECTIVITIES OF INHIBITORS OF ACETYLCHOLINESTERASE AND ITS MOLECULAR FORMS IN NORMAL AND ALZHEIMER’S DISEASE BRAIN* Z. RAKONCZAY** Alzheimer’s Disease Research Center, Department of Psychiatry, University of Szeged, Szeged, Hungary (Received: April 2, 2003; accepted: April 24, 2003) Eight inhibitors of acetylcholinesterase (AChE), tacrine, bis-tacrine, donepezil, rivastigmine, galanta- mine, heptyl-physostigmine, TAK-147 and metrifonate, were compared with regard to their effects on AChE and butyrylcholinesterase (BuChE) in normal human brain cortex. Additionally, the IC50 values of different molecular forms of AChE (monomeric, G1, and tetrameric, G4) were determined in the cerebral cortex in both normal and Alzheimer’s human brains. The most selective AChE inhibitors, in decreasing sequence, were in order: TAK-147, donepezil and galantamine. For BuChE, the most specific was rivastigmine. However, none of these inhibitors was absolutely specific for AChE or BuChE. Among these inhibitors, tacrine, bis-tacrine, TAK-147, metrifonate and galantamine inhibited both the G1 and G4 AChE forms equally well. Interestingly, the AChE molecular forms in Alzheimer samples were more sensitive to some of the inhibitors as compared with the normal samples. Only one inhibitor, rivastig- mine, displayed preferential inhibition for the G1 form of AChE. We conclude that a molecular form-spe- cific inhibitor may have therapeutic applications in inhibiting the G1 form, which is relatively unchanged