New Zealand Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Chemical Chelators As Reversal Agents for Drug
(19) TZZ_ _ZZZ_T (11) EP 1 210 090 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/724 (2006.01) A61K 31/194 (2006.01) 18.06.2014 Bulletin 2014/25 A61P 39/04 (2006.01) (21) Application number: 00964006.1 (86) International application number: PCT/EP2000/007694 (22) Date of filing: 07.08.2000 (87) International publication number: WO 2001/012202 (22.02.2001 Gazette 2001/08) (54) USE OF CHEMICAL CHELATORS AS REVERSAL AGENTS FOR DRUG- INDUCED NEUROMUSCULAR BLOCK VERWENDUNG VON CHEMISCHEN CHELATOREN ZUR UMKEHRUNG VON PHARMAKOLOGISCH-INDUZIERTER NEUROMUSKULÄRER BLOCKIERUNG UTILISATION D’AGENTS CHIMIQUES CHELATANTS COMME AGENTS DE NEUTRALISATION DU BLOCAGE NEUROMUSCULAIRE PROVOQUE PAR DES MEDICAMENTS (84) Designated Contracting States: (56) References cited: AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU AU-A- 3 662 895 MC NL PT SE • B DESIRE: "Inactivaton of sarin and soman by (30) Priority: 13.08.1999 EP 99306411 cyclodextrins in vitro" EXPERIENTIA, vol. 43, no. 4, 1987, pages 395-397, XP000907287 (43) Date of publication of application: • B. DESIRE: "Interaction of soman with beta- 05.06.2002 Bulletin 2002/23 cyclodextrin" FUNDAMENTAL AND APPLIED TOXICOLOGY, vol. 7, no. 4, 1986, pages 647-657, (73) Proprietor: Merck Sharp & Dohme B.V. XP000911170 2031 BN Haarlem (NL) • C. MAY: "Development of a toxin-binding agent as a treatment for tunicamycinuracil toxicity: (72) Inventors: protection against tunicamycin poisoning of • BOM, Antonius, Helena, Adolf sheep" AUSTRALIAN VETERINARY JOURNAL, Ratho, Midlothian EH28 8NY (GB) vol. 76, no. -

Comparative Study of Different Doses of Rocuronium Bromide For
MedDocs Publishers Annals of Anesthesia and Pain Medicine Open Access | Research Article Comparative study of different doses of rocuronium bromide for endotracheal intubation Nidhi V Sardhara1*; Sonal A Shah2; Dhaval P Pipaliya3; Shivansh Gupta3 1SVP hospital, 13th floor, Room no 13125, Near Ellis bridge, Ahmedabad, Gujarat-380006 2Assistant Professor, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India 3First year Resident, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India *Corresponding Author(s): Nidhi V Sardhara Abstract SVP hospital, 13th floor, Room no 13125, Near Ellis Background: Endotracheal intubation is one of such bridge, Ahmedabad, Gujarat-380006 development without which general anesthesia cannot be Tel: +91-9428-44-7878, Fax: 7926-57-8452 considered safe for any major surgery particularly head and Email: [email protected] neck, thoracic and abdominal surgeries. Ever since the ad- vent of anesthesia, anesthesiologists have been in search of an ideal muscle relaxants which can provide ideal intu- bating conditions in ultrashort duration with minimal side effects. Rocuronium bromide provides fast onset of action, Received: Mar 11, 2020 an intermediate duration of action and rapid recovery, good Accepted: Apr 24, 2020 to excellent intubating conditions at doses having minimal Published Online: Apr 30, 2020 or no haemodynamic changes. Present study is to compare the effect of different doses (0.6 mg/kg, 0.9 mg/kg, 1.2 mg/ Journal: Annals of Anesthesia and Pain Medicine kg) of Rocuronium bromide for endotracheal intubation at Publisher: MedDocs Publishers LLC 60 seconds. Online edition: http://meddocsonline.org/ Material and methods: This study was carried out by Copyright: © Sardhara NV (2020). -

Download Document
FAM001159-0001 intervals of 4-7 days to usual dose of 75-100 mg Dolmatil® (Sanofi-Synthelabo) ~ 100 rag/5 mL, thioridazine 100 rag/ per course and max. 4 injections; max. duration of daily according to response; CHILD not recom- Tablets, both scored, sulpiride 200 rag, net price ’ net price 300 mL = £7.14. Label: 2 treatment 2 weeks---if maintenance treatment mended 100-tabpack=£13.85;400mg(f/c), 100-tab ! fNote. These suspensions should not be diluted but the necessary change to an oral antipsychotic 2-3 Short-term adjunctive management of severe pack = £36¯29. Label: 2 .: :~t~a preparations may be mixed with each other to days after last injection, or to a longer acting anti- anxiety, 15-20rag daily in divided doses; max. Sulpltil® (Pharmacia) I~ ~0iovid¢ intermediate strengths psychotic depot injection given concomitantly 40 mg daily; CHILD not recommended Tablets, scored, sulpiride 200 rag. Net price 28-tab "ff~-~p, brown, thioridazine (as hydrochloride) with last injection of zuclopenthixol acetate; By deep intramuscular injection, psychoses, mania, pack = £4.29; 112-tab pack = £12.85. Label: 2 ~ff.~5"~mg/5 mL, net price 300 mL = £1.98. Label: 2 CHILD not recommended prochlorperazine mesilate 12.5-25 mg 2-3 times Sulpor® (Rosemont) IPoMI Clopi~ol Acuphase® (Lundbeck) daily; CHILD not recommended Oral solution, sugar-free, lemon- and aniseed-fla. LUOPERAZINE Injection (oily), zuclopenthixol acetate 50 mg/mL. By rectum in suppositories, psychoses, mania, the voured, sulpiride 200 mg/5 mL, net price 150 mL (’~’n~ications: see under Dose; anti-emetic (section Net price I-mL amp = £5.20; 2-mL amp = £10.03 equivalent of prochlorperazine maleate 25 mg 2- = £27.00. -

PMBJP Product.Pdf
Sr. Drug Generic Name of the Medicine Unit Size MRP Therapeutic Category No. Code Analgesic & Antipyretic / Muscle 1 1 Aceclofenac 100mg and Paracetamol 325 mg Tablet 10's 10's 8.00 relaxants Analgesic & Antipyretic / Muscle 2 2 Aceclofenac Tablets IP 100mg 10's 10's 4.37 relaxants Acetaminophen 325 + Tramadol Hydrochloride 37.5 film Analgesic & Antipyretic / Muscle 3 4 10's 8.00 coated Tablet 10's relaxants Analgesic & Antipyretic / Muscle 4 5 ASPIRIN Tablets IP 150 mg 14's 14's 2.70 relaxants DICLOFENAC 50 mg+ PARACETAMOL 325 mg+ Analgesic & Antipyretic / Muscle 5 6 10's 11.30 CHLORZOXAZONE 500 mg Tablets 10's relaxants Diclofenac Sodium 50mg + Serratiopeptidase 10mg Tablet Analgesic & Antipyretic / Muscle 6 8 10's 12.00 10's relaxants Analgesic & Antipyretic / Muscle 7 9 Diclofenac Sodium (SR) 100 mg Tablet 10's 10's 6.12 relaxants Analgesic & Antipyretic / Muscle 8 10 Diclofenac Sodium 25mg per ml Inj. IP 3 ml 3 ml 2.00 relaxants Analgesic & Antipyretic / Muscle 9 11 Diclofenac Sodium 50 mg Tablet 10's 10's 2.90 relaxants Analgesic & Antipyretic / Muscle 10 12 Etoricoxilb Tablets IP 120mg 10's 10's 33.00 relaxants Analgesic & Antipyretic / Muscle 11 13 Etoricoxilb Tablets IP 90mg 10's 10's 25.00 relaxants Analgesic & Antipyretic / Muscle 12 14 Ibuprofen 400 mg + Paracetamol 325 mg Tablet 10's 15's 5.50 relaxants Analgesic & Antipyretic / Muscle 13 15 Ibuprofen 200 mg film coated Tablet 10's 10's 1.80 relaxants Analgesic & Antipyretic / Muscle 14 16 Ibuprofen 400 mg film coated Tablet 10's 15's 3.50 relaxants Analgesic & Antipyretic -
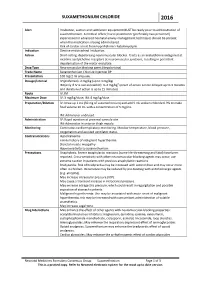
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

10 Mg/Ml Solution for Injection/Infusion Atracurium
Package leaflet: Information for the patient <Product name> 10 mg/ml solution for injection/infusion Atracurium besilate Read all of this leaflet carefully before you start using this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or nurse. - If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What <Product name> is and what it is used for 2. What you need to know before you receive <Product name> 3. How to use <Product name> 4. Possible side effects 5. How to store <Product name> 6. Contents of the pack and other information 1. What <Product name> is and what it is used for <Product name> belongs to a group of medicines called muscle relaxants. <Product name> is used during surgery to relax muscles and to assist with inserting a breathing tube and with artificial breathing. It is also used to help with artificial breathing at patients in intensive care units. 2. What you need to know before you receive <Product name> <Product name> cannot be administered: - if you are allergic to atracurium besilate, cisatracurium or any of the other ingredients of this medicine (listed in section 6). If you think that this applies to you talk to your doctor before <Product name> is administered to you. Warnings and precautions Talk to your doctor or nurse before using <Product name>: - if you have an allergy or bronchial -

(12) United States Patent (10) Patent N0.: US 7,265,099 B1 Born Et A1
US007265099B1 (12) United States Patent (10) Patent N0.: US 7,265,099 B1 Born et a1. (45) Date of Patent: *Sep. 4, 2007 (54) USE OF CHEMICAL CHELATORS AS Tarver, G. et al “2-O-Substituted cyclodextrins as reversal REVERSAL AGENTS FOR DRUG-INDUCED agents . ” Bioorg. Med. Chem. (2002) vol. 10, pp 1819-1827.* NEUROMUSCULAR BLOCK Zhang, M. “Drug-speci?c cyclodextrins . ” Drugs of the Future (2003) vol. 28, no 4, pp 347-354.* (75) Inventors: Antonius Helena Adolf Bom, Lee, C. “Structure, conformation, and action of neuromuscular Midlothian (GB); Alan William Muir, blocking drugs” Brit. J. Anesth. (2001) vol. 87, no 5, pp 755-769.* Lanark (GB); David Rees, Gothenburg B Desire: “Inactivation of sarin and soman by cyclodextrins in (SE) vitro” EXPERIENTIA, vol. 43, No. 4, 1987, pp. 395-397. B. Desire: “Interaction of soman with beta-cyclodextrin” Funda (73) Assignee: Organon N.V., Oss (NL) mental and Applied Toxicology, vol. 7, No. 4, 1986, pp. 647-657. ( * ) Notice: Subject to any disclaimer, the term of this C. May: “Development of a toxin-bindng agent as a treatment for patent is extended or adjusted under 35 tunicamycinuracil toxicity: protection against tunicamycin poison U.S.C. 154(b) by 0 days. ing of sheep” Australian Veterinary Journal, vol. 76, No. 11, 1998 pp. 752-756. This patent is subject to a terminal dis K. Uekama: “Effects of cyclodextrins on chlorpromaZine-induced claimer. haemolysis and nervous systems responses” J. Pharm. Pharmacol., vol. 33, No. 11, 1981, pp. 707-710. (21) Appl. No.: 10/049,393 T. Irie: “Protective mechanism of beta-cyclodextrin for the hemolysis induced With phenothiazine neuroleptics in vitro” J. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Zambia Essential Medicine List (Zeml) 03 2013
ZAMBIA ESSENTIAL MEDICINE LIST (ZEML) 03 2013 Drug Presentation Level VEN 1 Drugs used in anaesthesia 1.1. Drugs used in general anaesthesia 1.1.1 Intravenous and intramuscular anaesthetics 1.1.1.1 Ketamin injection 10mg/ml (10 ml) II-IV V 1.1.1.2 Thiopentone sodium powder for reconstitution 1g and 5g vials II-IV V 1.1.2 Inhalation anaesthetics 1.1.2.1 Halothane inhalation II-IV V 1.1.2.2 Nitrous oxide medical gas II-IV E 1.1.3 Muscle relaxants 1.1.3.1 Suxamethonium chloride injection 50mg/ml, (2ml) II-IV V 1.1.4 Anticholinesterases 1.1.4.1 Neostigmine injection 2.5mg/ml, (1ml) II-IV V 1.2 Drugs used in local anaesthesia 1.2.1. Lignocaine injection 1% (10ml, 50ml) I-IV V 1.2.2 Lignocaine + adrenaline dental cartridge injection 2% (1 in 80,000) II-IV V 1.3 Drugs used in spinal anaesthesia 1.3.1 Bupivacaine/glucose injection 0.5%, (4ml) IV E 1 Drug Presentation Level VEN 2. Drugs acting on the gastrointestinal system 2.1 Antacids 2.1.1 Aluminium hydroxide gel, chewable tablets I-IV E 2.12 Magnesium trisilicate Compound chewable tablets, mixture I-IV E 2.2. Antispasmodics 2.2.1 Hyoscine butyl bromide injection 20mg/ml, (1ml) II-IV E 2.2.2 Propantheline bromide tablets 15mg I-IV E 2.3 Ulcer healing drugs 2.3.1 Cimetidine tablets 200mg II-IV E 2.3.2 Omeprazole tablets 10mg, II-IV E 2.3.3 Ranitidine tablets 150mg II-IV E 2.3.4 Tripotassium dicitratobismuthate tablets 120mg II-IV E 2.3.5 Clarithromycin tablets 250mg II-IV E 2.4. -

2018 Product List Expertise in Injectables
2018 Product list Expertise in injectables Flucloxacillin ................................................................................ ................................................... 250 mg Antibiotics ................................................................................ ................................................... Flucloxacillin 500 mg Amoxicillin ....................................................................................... .................................................... 250 mg ................................................................................ .................................................................. Flucloxacillin 1 g Amoxicillin ....................................................................................... .................................................... 500 mg ..................................................................................... .................................................................. Fosfomycin 1 g Amoxicillin ....................................................................................... ................................................................... 1 g ..................................................................................... .................................................................. Fosfomycin 4 g Amoxicillin ....................................................................................... ................................................................... 2 g Gentamicin ...................................................................................... -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Murexal 10 mg/mL solution for injection in pre-filled syringe 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of solution for injection contains 10 mg of suxamethonium chloride anhydrous (as 11 mg of suxamethonium chloride dihydrate). Each 10 ml pre-filled syringe contains 100 mg of suxamethonium chloride anhydrous (as 110 mg of suxamethonium chloride dihydrate). Excipient with known effect: Each ml of solution for injection contains 2.79 mg equivalent to 0.12 mmol of sodium. Each 10 ml pre-filled syringe contains 27.9 mg equivalent to 1.2 mmol of sodium. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection (injection). Clear and colourless solution. pH: 3.0 – 4.5 Osmolality: 250-350 mOsm/Kg 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Murexal is indicated as a muscle relaxant to facilitate endotracheal intubation during induction of general anaesthesia or emergency situations, in adults and paediatric population above 12 years of age. 4.2 Posology and method of administration Suxamethonium should be administered only by or under close supervision of an experienced clinician (anaesthesist, intensivist, emergency physician) familiar with its action, characteristics and hazards, who is skilled in the management of intubation and artificial respiration and only where there are adequate facilities for immediate endotracheal intubation with administration of oxygen by intermittent positive pressure ventilation. It is given intravenously after anaesthesia has been induced and should not be administered to the conscious patient. Posology Adults To achieve endotracheal intubation, suxamethonium chloride is usually administered by bolus intravenous injection in a dose of 1 mg/kg body weight. -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known.