Suxamethoniumchlorideinj.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Study of Different Doses of Rocuronium Bromide For
MedDocs Publishers Annals of Anesthesia and Pain Medicine Open Access | Research Article Comparative study of different doses of rocuronium bromide for endotracheal intubation Nidhi V Sardhara1*; Sonal A Shah2; Dhaval P Pipaliya3; Shivansh Gupta3 1SVP hospital, 13th floor, Room no 13125, Near Ellis bridge, Ahmedabad, Gujarat-380006 2Assistant Professor, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India 3First year Resident, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India *Corresponding Author(s): Nidhi V Sardhara Abstract SVP hospital, 13th floor, Room no 13125, Near Ellis Background: Endotracheal intubation is one of such bridge, Ahmedabad, Gujarat-380006 development without which general anesthesia cannot be Tel: +91-9428-44-7878, Fax: 7926-57-8452 considered safe for any major surgery particularly head and Email: [email protected] neck, thoracic and abdominal surgeries. Ever since the ad- vent of anesthesia, anesthesiologists have been in search of an ideal muscle relaxants which can provide ideal intu- bating conditions in ultrashort duration with minimal side effects. Rocuronium bromide provides fast onset of action, Received: Mar 11, 2020 an intermediate duration of action and rapid recovery, good Accepted: Apr 24, 2020 to excellent intubating conditions at doses having minimal Published Online: Apr 30, 2020 or no haemodynamic changes. Present study is to compare the effect of different doses (0.6 mg/kg, 0.9 mg/kg, 1.2 mg/ Journal: Annals of Anesthesia and Pain Medicine kg) of Rocuronium bromide for endotracheal intubation at Publisher: MedDocs Publishers LLC 60 seconds. Online edition: http://meddocsonline.org/ Material and methods: This study was carried out by Copyright: © Sardhara NV (2020). -
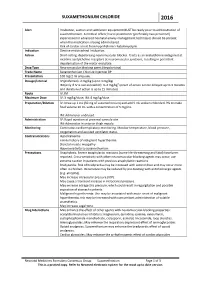
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Zambia Essential Medicine List (Zeml) 03 2013
ZAMBIA ESSENTIAL MEDICINE LIST (ZEML) 03 2013 Drug Presentation Level VEN 1 Drugs used in anaesthesia 1.1. Drugs used in general anaesthesia 1.1.1 Intravenous and intramuscular anaesthetics 1.1.1.1 Ketamin injection 10mg/ml (10 ml) II-IV V 1.1.1.2 Thiopentone sodium powder for reconstitution 1g and 5g vials II-IV V 1.1.2 Inhalation anaesthetics 1.1.2.1 Halothane inhalation II-IV V 1.1.2.2 Nitrous oxide medical gas II-IV E 1.1.3 Muscle relaxants 1.1.3.1 Suxamethonium chloride injection 50mg/ml, (2ml) II-IV V 1.1.4 Anticholinesterases 1.1.4.1 Neostigmine injection 2.5mg/ml, (1ml) II-IV V 1.2 Drugs used in local anaesthesia 1.2.1. Lignocaine injection 1% (10ml, 50ml) I-IV V 1.2.2 Lignocaine + adrenaline dental cartridge injection 2% (1 in 80,000) II-IV V 1.3 Drugs used in spinal anaesthesia 1.3.1 Bupivacaine/glucose injection 0.5%, (4ml) IV E 1 Drug Presentation Level VEN 2. Drugs acting on the gastrointestinal system 2.1 Antacids 2.1.1 Aluminium hydroxide gel, chewable tablets I-IV E 2.12 Magnesium trisilicate Compound chewable tablets, mixture I-IV E 2.2. Antispasmodics 2.2.1 Hyoscine butyl bromide injection 20mg/ml, (1ml) II-IV E 2.2.2 Propantheline bromide tablets 15mg I-IV E 2.3 Ulcer healing drugs 2.3.1 Cimetidine tablets 200mg II-IV E 2.3.2 Omeprazole tablets 10mg, II-IV E 2.3.3 Ranitidine tablets 150mg II-IV E 2.3.4 Tripotassium dicitratobismuthate tablets 120mg II-IV E 2.3.5 Clarithromycin tablets 250mg II-IV E 2.4. -

2018 Product List Expertise in Injectables
2018 Product list Expertise in injectables Flucloxacillin ................................................................................ ................................................... 250 mg Antibiotics ................................................................................ ................................................... Flucloxacillin 500 mg Amoxicillin ....................................................................................... .................................................... 250 mg ................................................................................ .................................................................. Flucloxacillin 1 g Amoxicillin ....................................................................................... .................................................... 500 mg ..................................................................................... .................................................................. Fosfomycin 1 g Amoxicillin ....................................................................................... ................................................................... 1 g ..................................................................................... .................................................................. Fosfomycin 4 g Amoxicillin ....................................................................................... ................................................................... 2 g Gentamicin ...................................................................................... -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Murexal 10 mg/mL solution for injection in pre-filled syringe 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of solution for injection contains 10 mg of suxamethonium chloride anhydrous (as 11 mg of suxamethonium chloride dihydrate). Each 10 ml pre-filled syringe contains 100 mg of suxamethonium chloride anhydrous (as 110 mg of suxamethonium chloride dihydrate). Excipient with known effect: Each ml of solution for injection contains 2.79 mg equivalent to 0.12 mmol of sodium. Each 10 ml pre-filled syringe contains 27.9 mg equivalent to 1.2 mmol of sodium. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection (injection). Clear and colourless solution. pH: 3.0 – 4.5 Osmolality: 250-350 mOsm/Kg 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Murexal is indicated as a muscle relaxant to facilitate endotracheal intubation during induction of general anaesthesia or emergency situations, in adults and paediatric population above 12 years of age. 4.2 Posology and method of administration Suxamethonium should be administered only by or under close supervision of an experienced clinician (anaesthesist, intensivist, emergency physician) familiar with its action, characteristics and hazards, who is skilled in the management of intubation and artificial respiration and only where there are adequate facilities for immediate endotracheal intubation with administration of oxygen by intermittent positive pressure ventilation. It is given intravenously after anaesthesia has been induced and should not be administered to the conscious patient. Posology Adults To achieve endotracheal intubation, suxamethonium chloride is usually administered by bolus intravenous injection in a dose of 1 mg/kg body weight. -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. TRACRIUM (atracurium besilate 10 mg/mL injections (2.5 mL and 5.0 mL)) 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mL ampoule contains 25 mg atracurium besilate, each 5 mL ampoule contains 50 mg atracurium besilate. TRACRIUM 2.5 mL and 5.0 mL injections contain no preservative. 3. PHARMACEUTICAL FORM TRACRIUM injection is a clear, faintly yellow, sterile, aqueous solution in a glass ampoule containing 10 mg/mL atracurium besilate. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications TRACRIUM is a highly selective, competitive or non-depolarising neuromuscular blocking agent which is used as an adjunct to general anaesthesia to enable tracheal intubation to be performed and to relax skeletal muscles during surgery or controlled ventilation, and to facilitate mechanical ventilation in Intensive Care Unit (ICU) patients. 4.2 Dose and method of administration Use in adults Injection TRACRIUM is administered by intravenous injection. The dosage range for adults is 0.3 to 0.6 mg/kg (depending on the duration of full block required) and will provide adequate relaxation for 15 to 35 minutes. Endotracheal intubation can usually be accomplished within 90 seconds from the intravenous injection of 0.5 to 0.6 mg/kg. Full block can be prolonged with supplementary doses of 0.1 to 0.2 mg/kg as required. Successive supplementary dosing does not give rise to accumulation of neuromuscular blocking effect. Spontaneous recovery from the end of full block occurs in about 35 minutes as measured by the restoration of the tetanic response to 95% of normal neuromuscular function. -

Pharmacology
STATE ESTABLISHMENT «DNIPROPETROVSK MEDICAL ACADEMY OF HEALTH MINISTRY OF UKRAINE» V.I. MAMCHUR, V.I. OPRYSHKO, А.А. NEFEDOV, A.E. LIEVYKH, E.V.KHOMIAK PHARMACOLOGY WORKBOOK FOR PRACTICAL CLASSES FOR FOREIGN STUDENTS STOMATOLOGY DEPARTMENT DNEPROPETROVSK - 2016 2 UDC: 378.180.6:61:615(075.5) Pharmacology. Workbook for practical classes for foreign stomatology students / V.Y. Mamchur, V.I. Opryshko, A.A. Nefedov. - Dnepropetrovsk, 2016. – 186 p. Reviewed by: N.I. Voloshchuk - MD, Professor of Pharmacology "Vinnitsa N.I. Pirogov National Medical University.‖ L.V. Savchenkova – Doctor of Medicine, Professor, Head of the Department of Clinical Pharmacology, State Establishment ―Lugansk state medical university‖ E.A. Podpletnyaya – Doctor of Pharmacy, Professor, Head of the Department of General and Clinical Pharmacy, State Establishment ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ Approved and recommended for publication by the CMC of State Establishment ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ (protocol №3 from 25.12.2012). The educational tutorial contains materials for practical classes and final module control on Pharmacology. The tutorial was prepared to improve self-learning of Pharmacology and optimization of practical classes. It contains questions for self-study for practical classes and final module control, prescription tasks, pharmacological terms that students must know in a particular topic, medical forms of main drugs, multiple choice questions (tests) for self- control, basic and additional references. This tutorial is also a student workbook that provides the entire scope of student’s work during Pharmacology course according to the credit-modular system. The tutorial was drawn up in accordance with the working program on Pharmacology approved by CMC of SE ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ on the basis of the standard program on Pharmacology for stomatology students of III - IV levels of accreditation in the specialties Stomatology – 7.110105, Kiev 2011. -

Troikaa Pharmaceuticals Ltd's Drugs List Anaesthetics
Troikaa Pharmaceuticals Ltd's Drugs List Anaesthetics Sr.No Product Name /Brand Name Strength/ Composition Presentation Dosage Form Choline Salicylate Solution equivalent to Choline Salicylate 8.7% w/w + 1 ALCINIL 10GM TUBE 1 TUBE TUBE BenzalkoniumChloride solution 0.01%w/w + Lignocaine Hcl 2% w/w Bupivacaine HCl Preservative 2 Free 0.5% Inj. 5 mg / ml 20 ml Vial Injection BUPIPTROY PF Bupivacaine HCl 0.5% Inj. 3 5 mg / ml 20 ml Vial Injection BUPITROY Bupivacaine HCl in Dextrose 4 Inj. 20 mg + 320 mg 5 X 4 ml Amp Injection BUPITROY-HEAVY 5 ml & 10 ml Midazolam Inj. a) 1 mg / ml 5 vial Injection BENZOSED b) 5 mg / ml 3 ml Amp Dexmedetomidine 50 mcg/0.5 6 DEXIT 50 1X0.5 ML 1X0.5 ML AMPOULE ml 7 DEXIT 100 1X1 ML Dexmedetomidine 100 mcg/1 ml 1X1 ML AMPOULE 8 DEXIT 200 1X2 ML Dexmedetomidine 200 mcg/2 ml 1X2 ML AMPOULE Isoflurane 100 ml / 250 ml Volatile liquid 9 ISOTROY 100 ml & 250 ml Bottle for Inhalation 10 ISOTROY 30ML 1X30ML Isoflurane 30 Ml 1X30ML BOTTLE Ketamine Inj. 11 KETAMAX-50 50 mg / ml 10 ml Vial Injection Rocuronium Nbromide Inj. 12 10 mg / ml 5 ml Vial Injection ROCUTROY 50 ml / 250 ml Sevoflurane Volatile liquid 13 50 ml & 250 ml Bottle SEVOTROY for Inhalation Suxamethonium Chloride Each ml contains 10 ml clear glass 14 Injection B.p Suxamethonium Chloride 50 mg Injection vial SUXATROY Propofol Inj. 20 ml / 50 ml 15 10mg / ml - 20 ml / 50 ml Vial Injection TROYPOFOL Vial Propofol MCT Inj. -

Summary of Product Characteristics 1 Name Of
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT [Invented name] 50mg/ml Solution for Injection or Infusion. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION [Invented name] 50mg/ml (100mg/2ml). Each ml of solution for injection or infusion contains 50 mg of suxamethonium chloride dihydrate (equivalent to 36.55 mg of suxamethonium). Each 2 ml ampoule contains 100 mg suxamethonium chloride dihydrate (equivalent to 73.1 mg of suxamethonium). For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for Injection or Infusion. Clear, colourless solution. pH of the solution 3.0-4.2. Osmolality of the product is 300-365 mOsmol/kg. 4 CLINICAL PARTICULARS 4.1 Therapeutic indications Used for muscle relaxation during general anaesthesia . 4.2 Posology and method of administration Use by intravenous injection Adults and Children over 12 years The dose is dependent on body weight, the degree of muscular relaxation required, the route of administration, and the response of individual patients. To achieve endotracheal intubation Suxamethonium Chloride is usually administered intravenously in a dose of 1mg/kg. This dose will usually produce muscular relaxation in about 30 to 60 seconds and has a duration of action of about 2 to 6 minutes. Larger doses will produce more prolonged muscular relaxation, but doubling the dose does not necessarily double the duration of relaxation. Supplementary doses of Suxamethonium Chloride of 50% to 100% of the initial dose administered at 5 to 10 minute intervals will maintain muscle relaxation during short surgical procedures performed under general anaesthesia. The total dose of Suxamethonium Chloride should not exceed 500mg. -

II.4.6 Muscle Relaxants by Mayumi Nishikawa and Hitoshi Tsuchihashi
4.6 II.4.6 Muscle relaxants by Mayumi Nishikawa and Hitoshi Tsuchihashi Introduction Muscle relaxants can be classifi ed into the peripheral-and central-acting drug types (> Table 6.1). Th e drugs of the peripheral type are also called neuromuscular blocking agents. Th e peripheral- acting muscle relaxants are being used for muscle relaxation upon endotracheal intubation and/or general anaesthesia for surgical operation. Th e central-acting muscle relaxants are used for treat- ments of painful muscle contracture caused by locomotorial disorders, and for relaxation of mus- cle stiff ness caused by psychotic tension or by neurosis. Th e cases of poisoning by the peripheral- acting muscle relaxants oft en take place, because of its strong suppressive action on respiration. However, their poisoning is rare at the scene of medical treatments, because the risk is usually avoided by artifi cial respiration. Most of poisoning incidents due to the peripheral-acting muscle relaxants are intentional and/or homicidal. In this chapter, therefore, analytical methods only for the peripheral-acting muscle relaxants (> Table 6.2) are dealt with. Since these drugs are quater- nary ammonium salts, special care should be taken for their stability and effi cient extraction [1]. TLC analysis Reagents and its preparation • Tubocurarine chloride, suxamethonium chloride ( succinylcholine chloride) and pan- curonium bromide can be purchased from Sigma (St. Louis, MO, USA). For vecuronium bromide, pure powder is not commercially available; ampoule solution for -

Chapter 13 – Pharmacology of Muscle Relaxants and Their Antagonists Mohamed Naguib, Cynthia A
Chapter 13 – Pharmacology of Muscle Relaxants and Their Antagonists Mohamed Naguib, Cynthia A. Lien HISTORY AND CLINICAL USE In 1942 Griffith and Johnson[1] suggested that d-tubocurarine (dTc) is a safe drug to use during surgery to provide skeletal muscle relaxation. One year later, Cullen[2] described its use in 131 patients who had received general anesthesia for their surgery. In 1954, Beecher and Todd[3] reported a sixfold increase in mortality in patients receiving dTc versus those who had not received a relaxant. The increased mortality was due to a general lack of understanding of the pharmacology of neuromuscular blockers and their antagonism. The impact of residual neuromuscular blockade postoperatively was not appreciated, guidelines for monitoring muscle strength had not been established, and the importance of pharmacologically antagonizing residual blockade was not understood. Since then, the understanding of neuromuscular blocker pharmacology has improved, and relaxants have become an important component of many anesthetics and have facilitated the growth of surgery into new areas with the use of innovative techniques.[4] Succinylcholine, introduced by Thesleff[5] and by Foldes and colleagues in 1952,[4] changed anesthetic practice drastically. Its rapid onset of effect and ultrashort duration of action allowed for rapid tracheal intubation. In 1967, Baird and Reid first reported on clinical administration of the synthetic aminosteroid pancuronium.[6] Though similar to dTc, in terms of its duration of action, this compound had an improved cardiovascular side effect profile. It lacked ganglionic-blocking and histamine-releasing properties and was mildly vagolytic. The resulting increases in heart rate and blood pressure were considered significant improvements over its predecessors.