Pancuronium Bromide—A New Non-Depolarizing Muscle Relaxant
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Clinical Evaluation of Pipecuronium Bromide and Its Comparison With
Scholars Journal of Applied Medical Sciences (SJAMS) ISSN 2320-6691 (Online) Sch. J. App. Med. Sci., 2013; 1(6):943-950 ISSN 2347-954X (Print) ©Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com Research Article Clinical Evaluation of Pipecuronium Bromide and its Comparison with Pancuronium Bromide Kaushal RP Associate Professor, Department of cardiac anesthesia, Gandhi Medical College, Bhopal, India. *Corresponding author Kaushal RP Email: Abstract: The study was carried out to compare the intubating conditions, cardiovascular responses, neuro-muscular blocking properties and reversal characteristics of pipecuronium bromide and pancuronium bromide. This is a prospective hospital based study. 100 patients belonging to ASA grade I or II physical status aged 18 to 70 years were divided into two groups of 50 patients each. Group 1 received pipecuronium bromide in the dose of .08 mg / kg and group 2 patients received pancuronium bromide in dose of 0.1 mg/kg. Each patient was pre medicated uniformly. Time for onset of apnoea for pipecuronium and pancuronium were 91.64+ 3.59 sec. and 118.84 + 12.53 sec. respectively. The mean time for intubation was 126.60 +12.55 sec. and 144.60 + 22.87 sec. with pipecuronium and pancuronium respectively. Mean duration of block for pipecuronium was 78.64 + 8.97 min. the block for pancuronium lasted from +36-40 min with a mean duration of block 41.60+ 5.57 min. The mean duration of maintenance dose in pipecuronium cases was 45.08 + 7.19 min., while it was 27.06 + 5.01 min in pancuronium cases. -
Pancuronium Bromide (Pavulon | Evaluation of Its Clinical Pharmacology*
PANCURONIUM BROMIDE (PAVULON | EVALUATION OF ITS CLINICAL PHARMACOLOGY* ALLEN B. DOBKIN, M.D., WILLIAM EVEBS,M.D., SION GHANOONI, M.D., ASHLEYA. LEVY, VH.D., AND EDWARDT. THOMAS,M.B. PANCURONIUMBROMIDE IS AN amino steroid muscle relaxant (Figure 1) which was synthesized in 1964 by Hewett and Savage and has been studied and evaluated clinically in Europe during the past four years2-5 It is an odourless, white, crystal- line powder with a bitter astringent taste, melts at 215~ with decomposition, and is soluble in 50 parts of chloroform and one part water at 20~ The co]ourless solution is stable while sealed, but breaks down in a few hours after exposure to air. In Europe it is available in 2-ml ampoules containing 4 mg pancuronium bro- mide, 18 nag sodium chloride B.P. and water for injection B.P. to 2 mls. The prepara- tion which was used in this study contains preservatives (acetic acid and sodium acetate) to buffer the solution to pH 4.0. OOC.CH 3N~~ 2BP- CH3CO0 H H20 -- Pancur'ontum Bromide - Pavuton (R) FIGURE 1. Structural formula for paneuronium bromide - Pavulon| Pharmacological studies have shown that it has no hormonal action but is a potent non-depolarizing skeletal muscle relaxant like'tubocurarine and gallamine. It has a more rapid onset of action than tubocurarine with a similar duration of action. It has a somewhat longer action than gallamine. It has no significant effect on the blood pressure or the tracheobronchial tree due to the very. slight ganglion- blocking action and the claim is that no histamine is released, a It does not affect *From the Department of Anesthesiology, State University Itospital, State University of New York, Upstate Medical Center, Syracuse, New York, 13210, U.S.A. -

Comparative Study of Different Doses of Rocuronium Bromide For
MedDocs Publishers Annals of Anesthesia and Pain Medicine Open Access | Research Article Comparative study of different doses of rocuronium bromide for endotracheal intubation Nidhi V Sardhara1*; Sonal A Shah2; Dhaval P Pipaliya3; Shivansh Gupta3 1SVP hospital, 13th floor, Room no 13125, Near Ellis bridge, Ahmedabad, Gujarat-380006 2Assistant Professor, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India 3First year Resident, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India *Corresponding Author(s): Nidhi V Sardhara Abstract SVP hospital, 13th floor, Room no 13125, Near Ellis Background: Endotracheal intubation is one of such bridge, Ahmedabad, Gujarat-380006 development without which general anesthesia cannot be Tel: +91-9428-44-7878, Fax: 7926-57-8452 considered safe for any major surgery particularly head and Email: [email protected] neck, thoracic and abdominal surgeries. Ever since the ad- vent of anesthesia, anesthesiologists have been in search of an ideal muscle relaxants which can provide ideal intu- bating conditions in ultrashort duration with minimal side effects. Rocuronium bromide provides fast onset of action, Received: Mar 11, 2020 an intermediate duration of action and rapid recovery, good Accepted: Apr 24, 2020 to excellent intubating conditions at doses having minimal Published Online: Apr 30, 2020 or no haemodynamic changes. Present study is to compare the effect of different doses (0.6 mg/kg, 0.9 mg/kg, 1.2 mg/ Journal: Annals of Anesthesia and Pain Medicine kg) of Rocuronium bromide for endotracheal intubation at Publisher: MedDocs Publishers LLC 60 seconds. Online edition: http://meddocsonline.org/ Material and methods: This study was carried out by Copyright: © Sardhara NV (2020). -
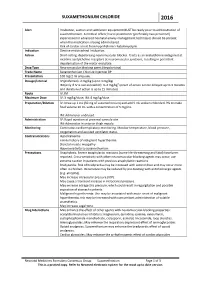
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Zambia Essential Medicine List (Zeml) 03 2013
ZAMBIA ESSENTIAL MEDICINE LIST (ZEML) 03 2013 Drug Presentation Level VEN 1 Drugs used in anaesthesia 1.1. Drugs used in general anaesthesia 1.1.1 Intravenous and intramuscular anaesthetics 1.1.1.1 Ketamin injection 10mg/ml (10 ml) II-IV V 1.1.1.2 Thiopentone sodium powder for reconstitution 1g and 5g vials II-IV V 1.1.2 Inhalation anaesthetics 1.1.2.1 Halothane inhalation II-IV V 1.1.2.2 Nitrous oxide medical gas II-IV E 1.1.3 Muscle relaxants 1.1.3.1 Suxamethonium chloride injection 50mg/ml, (2ml) II-IV V 1.1.4 Anticholinesterases 1.1.4.1 Neostigmine injection 2.5mg/ml, (1ml) II-IV V 1.2 Drugs used in local anaesthesia 1.2.1. Lignocaine injection 1% (10ml, 50ml) I-IV V 1.2.2 Lignocaine + adrenaline dental cartridge injection 2% (1 in 80,000) II-IV V 1.3 Drugs used in spinal anaesthesia 1.3.1 Bupivacaine/glucose injection 0.5%, (4ml) IV E 1 Drug Presentation Level VEN 2. Drugs acting on the gastrointestinal system 2.1 Antacids 2.1.1 Aluminium hydroxide gel, chewable tablets I-IV E 2.12 Magnesium trisilicate Compound chewable tablets, mixture I-IV E 2.2. Antispasmodics 2.2.1 Hyoscine butyl bromide injection 20mg/ml, (1ml) II-IV E 2.2.2 Propantheline bromide tablets 15mg I-IV E 2.3 Ulcer healing drugs 2.3.1 Cimetidine tablets 200mg II-IV E 2.3.2 Omeprazole tablets 10mg, II-IV E 2.3.3 Ranitidine tablets 150mg II-IV E 2.3.4 Tripotassium dicitratobismuthate tablets 120mg II-IV E 2.3.5 Clarithromycin tablets 250mg II-IV E 2.4. -

Clinical Pharmacokinetics of Muscle Relaxants
i ,I .. / ,""- Clinical Pharmacokinetics 2: 330-343 (1977) © ADIS Press 1977 Clinical Pharmacokinetics of Muscle Relaxants L.B. Wingard and DR. Cook Departments of Pharmacology and Anesthesiology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania SUl1lmary Muscle relaxants are commonly used as an adjunct to general anaesthesia and to facilitate ventilator care in the intensive care unit. The muscle relaxants are unique in that the degree of neurol1luscular blockade can be directly measured. Thus, for some of the muscle relaxants it is possible to correlate the degree of neuromuscular blockade with the plasma concentration of drug. This quantilalive pllGrmacokinelic approach has been applied primarily to d tubocurarine and to a lesser extent to suxamethonium (succinylcholine), gallamine and pan curoniul1l. The pharl1lacokinetic information for the other relaxants is mostly descriptive and incomplete. The variation in drug concentration over time is in.fluenced by the distribution, metabolism and excretion of drug. Metabolism by plasma cholinesterase plays a maJor role in the termina tion (~f action of suxamethonium. Although pancuronium is partly metabolised its major metabolites have moderate pharmacological activity. The other relaxants are excreted through the kidney. For gallamine and dimethyl-tubocurarine, renal excretion appears to be the only means qf eliinination. However, biliary excretion probably provides an alternative route of elimination for d-tubocurarine and pancuronium. In patients with impaired renal function the duration qf neuromusClilar blockade may be markedly prolonged following standard doses of gallamine or dimethyl-tubocurarine, may be slightly prolonged following standard doses of pancumnium, and is near normal following standard doses qf d-tubocurarine. Following large or repeated doses q(pancuronium or d-tubocurarine, the duration q{ neuromuscular blockade may be markedly prolonged. -

2018 Product List Expertise in Injectables
2018 Product list Expertise in injectables Flucloxacillin ................................................................................ ................................................... 250 mg Antibiotics ................................................................................ ................................................... Flucloxacillin 500 mg Amoxicillin ....................................................................................... .................................................... 250 mg ................................................................................ .................................................................. Flucloxacillin 1 g Amoxicillin ....................................................................................... .................................................... 500 mg ..................................................................................... .................................................................. Fosfomycin 1 g Amoxicillin ....................................................................................... ................................................................... 1 g ..................................................................................... .................................................................. Fosfomycin 4 g Amoxicillin ....................................................................................... ................................................................... 2 g Gentamicin ...................................................................................... -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Murexal 10 mg/mL solution for injection in pre-filled syringe 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of solution for injection contains 10 mg of suxamethonium chloride anhydrous (as 11 mg of suxamethonium chloride dihydrate). Each 10 ml pre-filled syringe contains 100 mg of suxamethonium chloride anhydrous (as 110 mg of suxamethonium chloride dihydrate). Excipient with known effect: Each ml of solution for injection contains 2.79 mg equivalent to 0.12 mmol of sodium. Each 10 ml pre-filled syringe contains 27.9 mg equivalent to 1.2 mmol of sodium. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection (injection). Clear and colourless solution. pH: 3.0 – 4.5 Osmolality: 250-350 mOsm/Kg 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Murexal is indicated as a muscle relaxant to facilitate endotracheal intubation during induction of general anaesthesia or emergency situations, in adults and paediatric population above 12 years of age. 4.2 Posology and method of administration Suxamethonium should be administered only by or under close supervision of an experienced clinician (anaesthesist, intensivist, emergency physician) familiar with its action, characteristics and hazards, who is skilled in the management of intubation and artificial respiration and only where there are adequate facilities for immediate endotracheal intubation with administration of oxygen by intermittent positive pressure ventilation. It is given intravenously after anaesthesia has been induced and should not be administered to the conscious patient. Posology Adults To achieve endotracheal intubation, suxamethonium chloride is usually administered by bolus intravenous injection in a dose of 1 mg/kg body weight. -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. TRACRIUM (atracurium besilate 10 mg/mL injections (2.5 mL and 5.0 mL)) 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mL ampoule contains 25 mg atracurium besilate, each 5 mL ampoule contains 50 mg atracurium besilate. TRACRIUM 2.5 mL and 5.0 mL injections contain no preservative. 3. PHARMACEUTICAL FORM TRACRIUM injection is a clear, faintly yellow, sterile, aqueous solution in a glass ampoule containing 10 mg/mL atracurium besilate. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications TRACRIUM is a highly selective, competitive or non-depolarising neuromuscular blocking agent which is used as an adjunct to general anaesthesia to enable tracheal intubation to be performed and to relax skeletal muscles during surgery or controlled ventilation, and to facilitate mechanical ventilation in Intensive Care Unit (ICU) patients. 4.2 Dose and method of administration Use in adults Injection TRACRIUM is administered by intravenous injection. The dosage range for adults is 0.3 to 0.6 mg/kg (depending on the duration of full block required) and will provide adequate relaxation for 15 to 35 minutes. Endotracheal intubation can usually be accomplished within 90 seconds from the intravenous injection of 0.5 to 0.6 mg/kg. Full block can be prolonged with supplementary doses of 0.1 to 0.2 mg/kg as required. Successive supplementary dosing does not give rise to accumulation of neuromuscular blocking effect. Spontaneous recovery from the end of full block occurs in about 35 minutes as measured by the restoration of the tetanic response to 95% of normal neuromuscular function. -

Inhibitory Actions of Vecuronium Bromide on Acetylcholine and Glutamate Responses at the Frog and Crayfish Neuromuscular Junction
Inhibitory Actions of Vecuronium Bromide on Acetylcholine and Glutamate Responses at the Frog and Crayfish Neuromuscular Junction Haruhiko SHINOZAKI and Michiko ISHIDA The Tokyo Metropolitan Institute of Medical Science, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113, Japan Accepted November 7, 1983 Abstract-Effects of vecuronium bromide, an analog of pancuronium, on the cholinergic and glutamatergic neuromuscular junction were investigated. Vecuronium depressed the postsynaptic response of the frog end-plate at lower concentrations than 10-6 g/ml without affecting the presynaptic events. Vecuronium decreased the amplitude of the double ACh potential, but the second potential was more markedly reduced than the first. In analogy with d-tubocurarine, this suggests that vecuronium may act in part as an open channel blocker at the frog end-plate. Vecuro nium depressed both the glutamate response and the excitatory junctional potential at the crayfish neuromuscular junction, although high concentrations were required. The drug increased the decay rate of extracellularly recorded excitatory functional potentials at the crayfish neuromuscular junction. The reduction of the crayfish synaptic response caused by vecuronium can be explained by the open channel blocking action at this functional site. The problem that cholinergic antagonists possess a property of channel blocking at the other transmitter system was discussed. Vecuronium is an analog of pancuronium, The neuromuscular blocking action of pan but is not the bis-quarternary ammonium, one curonium has been well documented (2). quarternary moiety of pancuronium being The comparison of pharmacological activities replaced by a tertiary amine (Fig. 11. Although of vecuronium to pancuronium was already it is known that replacement of one quar done in some earlier studies (3-6), and ternary moiety of the bis-quarternary am the effectiveness of both drugs is almost monium structure by a primary amine group the same, however, electrophysiological results in a considerable loss of potency (1), studies are lacking. -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Pancuronium Bromide 2 mg/ml Solution for Injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 1 ml contains 2 mg of pancuronium bromide. Each 2 ml ampoule contains 4 mg of pancuronium bromide. Excipients with known effect: For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection. A clear, colourless solution with a pH of 3.8 – 4.2. 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Pancuronium bromide is a neuromuscular blocking agent with a long duration of action. It is used as an adjuvant in surgical anaesthesia to obtain relaxation of the skeletal muscles in a wide range of surgical procedures. It is also used as relaxant in orthopaedic manipulations. Used in intensive care therapy for a variety of pathologies e.g. intractable status asthmaticus and tetanus. 4.2 Posology and method of administration Posology The use of a peripheral nerve stimulator is recommended for monitoring the neuromuscular block and recovery. The following may be used as a guideline: ADULT: Initial dose: 50-80 micrograms/kg (intubation within 150 to 120 seconds) Or 80-100 micrograms/kg (intubation accomplished within 120-90 seconds). Incremental doses: 10-20 micrograms/kg PAEDIATRIC: Initial dose: 60-100 micrograms/kg Incremental doses: 10-20 micrograms/kg NEONATES: Initial dose: 30-40 micrograms/kg Incremental doses: As neonates are sensitive this dose should be adjusted according to the initial response but generally incremental doses lie in the range 10 to 20 micrograms/kg. Ifsuxamethonium is used for intubation, the administration of pancuronium bromide should be delayed until the patient has clinically recovered from the neuromuscular block induced by suxamethonium.