Zambia Essential Medicine List (Zeml) 03 2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Steroid Recognition and Regulation of Hormone Action: Crystal
Research Article 799 Steroid recognition and regulation of hormone action: crystal structure of testosterone and NADP+ bound to 3a-hydroxysteroid/ dihydrodiol dehydrogenase Melanie J Bennett1†, Ross H Albert1, Joseph M Jez1, Haiching Ma2, Trevor M Penning2 and Mitchell Lewis1* Background: Mammalian 3a-hydroxysteroid dehydrogenases (3a-HSDs) Addresses: 1Department of Biochemistry and modulate the activities of steroid hormones by reversibly reducing their C3 Biophysics, The Johnson Research Foundation, 37th and Hamilton Walk, Philadelphia, PA 19104- ketone groups. In steroid target tissues, 3a-HSDs act on 5a-dihydrotestosterone, 6059, USA and 2Department of Pharmacology, a potent male sex hormone (androgen) implicated in benign prostate hyperplasia University of Pennsylvania School of Medicine, 37th and prostate cancer. Rat liver 3a-HSD belongs to the aldo-keto reductase (AKR) and Hamilton Walk, Philadelphia, PA 19104-6084, superfamily and provides a model for mammalian 3a-, 17b- and 20a-HSDs, USA. which share > 65% sequence identity. The determination of the structure of 3a- †Present address: Division of Biology, California + HSD in complex with NADP and testosterone (a competitive inhibitor) will help Institute of Technology, 1200 E California Blvd., to further our understanding of steroid recognition and hormone regulation by Pasadena, CA 91125, USA. mammalian HSDs. *Corresponding author. E-mail: [email protected] Results: We have determined the 2.5 Å resolution crystal structure of recombinant rat liver 3a-HSD complexed with NADP+ and testosterone. The Key words: aldo-keto reductase, crystal structure, structure provides the first picture of an HSD ternary complex in the AKR 3a-hydroxysteroid/dihydrodiol dehydrogenase, superfamily, and is the only structure to date of testosterone bound to a protein. -

Comparative Study of Different Doses of Rocuronium Bromide For
MedDocs Publishers Annals of Anesthesia and Pain Medicine Open Access | Research Article Comparative study of different doses of rocuronium bromide for endotracheal intubation Nidhi V Sardhara1*; Sonal A Shah2; Dhaval P Pipaliya3; Shivansh Gupta3 1SVP hospital, 13th floor, Room no 13125, Near Ellis bridge, Ahmedabad, Gujarat-380006 2Assistant Professor, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India 3First year Resident, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India *Corresponding Author(s): Nidhi V Sardhara Abstract SVP hospital, 13th floor, Room no 13125, Near Ellis Background: Endotracheal intubation is one of such bridge, Ahmedabad, Gujarat-380006 development without which general anesthesia cannot be Tel: +91-9428-44-7878, Fax: 7926-57-8452 considered safe for any major surgery particularly head and Email: [email protected] neck, thoracic and abdominal surgeries. Ever since the ad- vent of anesthesia, anesthesiologists have been in search of an ideal muscle relaxants which can provide ideal intu- bating conditions in ultrashort duration with minimal side effects. Rocuronium bromide provides fast onset of action, Received: Mar 11, 2020 an intermediate duration of action and rapid recovery, good Accepted: Apr 24, 2020 to excellent intubating conditions at doses having minimal Published Online: Apr 30, 2020 or no haemodynamic changes. Present study is to compare the effect of different doses (0.6 mg/kg, 0.9 mg/kg, 1.2 mg/ Journal: Annals of Anesthesia and Pain Medicine kg) of Rocuronium bromide for endotracheal intubation at Publisher: MedDocs Publishers LLC 60 seconds. Online edition: http://meddocsonline.org/ Material and methods: This study was carried out by Copyright: © Sardhara NV (2020). -
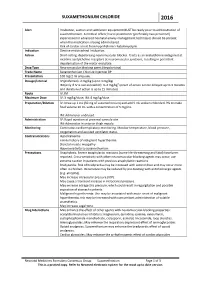
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

Apigenin-Loaded Solid Lipid Nanoparticle Attenuates Diabetic Nephropathy Induced by Streptozotocin Nicotinamide Through Nrf2/ HO-1/NF-Kb Signalling Pathway
International Journal of Nanomedicine Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Apigenin-Loaded Solid Lipid Nanoparticle Attenuates Diabetic Nephropathy Induced by Streptozotocin Nicotinamide Through Nrf2/ HO-1/NF-kB Signalling Pathway This article was published in the following Dove Press journal: International Journal of Nanomedicine Pingping Li1 Background: Apigenin is known to have a broad-spectrum efficacy in oxidative stress and Syed Nasir Abbas Bukhari 2 conditions due to inflammation, although weak absorption, fast metabolic rate and a fast Tahseen Khan 3 elimination (systemic) limit the pharmacological efficacy of this drug. Hence, we propose the Renukaradhya Chitti4 usage of highly bioavailable Apigenin-solid lipid nanoparticles (SLNPs) to recognize such Davan B Bevoor5 limitations. The defensive function of Apigenin-SLNPs on renal damage induced by strep 6 tozotocin (STZ) in animals was studied. Anand R Hiremath −1 7 Materials and Methods: We initially injected the rats with 35 mg kg streptozocin intraper Nagaraja SreeHarsha −1 8 itoneally, and after 7 days, the rats were then injected 150 mg kg of metformin intragastrically Yogendra Singh followed by a once-daily intragastric dose of Apigenin-SLNP (25 or 50 mg kg−1) for 9 Kumar Shiva Gubbiyappa a continuous period of 30 days. We then measured the level of insulin and blood glucose, 1Department of Nephrology, Zhengzhou superoxide dismutase, catalase and malondialdehyde in the tissues of the kidney. We also Central Hospital Affiliated to Zhengzhou observed messenger-RNA expression of Interleukin-1β, Interleukin-6 and Tumor Necrosis University, Zhengzhou City, Henan Province 450007, People’s Republic of Factor-alpha in renal tissue through RT-PCR technique. -

Men's Health& Testosterone
Q&A: NEW ENDOCRINE REVIEWS EIC DANIEL J. DRUCKER, MD JUNE 2018 THE LEADING MAGAZINE FOR ENDOCRINOLOGISTS Men’s Health & Testosterone ● New Endocrine Society guidelines detail why hypogonadism patients should be treated when consistently low T levels are tied to symptoms, not what the patient saw on television. ● A closer look at the T Trials shows that testosterone therapy may actually be suitable for older patients with hypogonadism. ONCE A DAY: ENS New research may finally achieve the “male pill” M ’ WHY ENDOCRINOLOGY?: A newcomer’s perspective from the bench HEALTH & TESTOSTERONE TEST YOUR KNOWLEDGE WITH PEDIATRIC ESAPTM THE LEADING MAGAZINE FOR ENDOCRINOLOGISTS 2017-2018 2017 – 2019 EDITORIAL ADVISORY BOARD Henry Anhalt, DO Bergen County Pediatric Endocrinology Chair, Hormone Health Network VP, Medical Affairs, Science 37 Sally Camper, PhD Department of Human Genetics University of Michigan Medical School Rodolfo J. Galindo, MD Assistant Professor of Medicine Mount Sinai School of Medicine Christian M. Girgis, MBBS, PhD, FRACP Royal North Shore and Westmead Hospitals University of Sydney, Australia Andrea Gore, PhD Division of Pharmacology and Toxicology University of Texas Daniel A. Gorelick, PhD Solve 100 New Cases In Department of Pharmacology & Toxicology University of Alabama at Birmingham One Module, Now Delivering: M. Carol Greenlee, MD, FACP Interactive online modules Western Slope Endocrinology Grand Junction, Colo. and printed reference book (Faculty for Transforming Clinical Practice initiative [TCPi]) Peer-review comparisons Gary D. Hammer, MD, PhD for each question Millie Schembechler Professor of Adrenal Cancer, Endocrine Oncology Program Detailed overall University of Michigan performance report Robert W. Lash, MD Chief Professional & Clinical Officer, Endocrine Society Lab values in conventional and SI Units Karl Nadolsky, DO Diabetes Obesity & Metabolic Institute 40 ABP MOC Part 2 points and Walter Reed National Military Medical Center; Uniformed Services University 40.0 AMA PRA Category 1 Credits™ Joshua D. -

Flavonoid Apigenin Is an Inhibitor of the NAD+Ase CD38: Implications for Cellular NAD+ Metabolism, Protein Acetylation, and Treatment of Metabolic Syndrome
Flavonoid Apigenin Is an Inhibitor of the NAD+ase CD38: Implications for Cellular NAD+ Metabolism, Protein Acetylation, and Treatment of Metabolic Syndrome The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Escande, Carlos, Veronica Nin, Nathan L. Price, Verena Capellini, Ana P. Gomes, Maria Thereza Barbosa, Luke O’Neil, Thomas A. White, David A. Sinclair, and Eduardo N. Chini. 2013. “Flavonoid Apigenin Is an Inhibitor of the NAD+ase CD38: Implications for Cellular NAD+ Metabolism, Protein Acetylation, and Treatment of Metabolic Syndrome.” Diabetes 62 (4): 1084-1093. doi:10.2337/ db12-1139. http://dx.doi.org/10.2337/db12-1139. Published Version doi:10.2337/db12-1139 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:12152908 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ORIGINAL ARTICLE Flavonoid Apigenin Is an Inhibitor of the NAD+ase CD38 Implications for Cellular NAD+ Metabolism, Protein Acetylation, and Treatment of Metabolic Syndrome Carlos Escande,1 Veronica Nin,1 Nathan L. Price,2 Verena Capellini,1 Ana P. Gomes,2 Maria Thereza Barbosa,1 Luke O’Neil,1 Thomas A. White,1 David A. Sinclair,2 and Eduardo N. Chini1 Metabolic syndrome is a growing health problem worldwide. It is syndrome (5). This concept was later expanded by others therefore imperative to develop new strategies to treat this using different approaches, including inhibition of poly-ADP- + pathology. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

2018 Product List Expertise in Injectables
2018 Product list Expertise in injectables Flucloxacillin ................................................................................ ................................................... 250 mg Antibiotics ................................................................................ ................................................... Flucloxacillin 500 mg Amoxicillin ....................................................................................... .................................................... 250 mg ................................................................................ .................................................................. Flucloxacillin 1 g Amoxicillin ....................................................................................... .................................................... 500 mg ..................................................................................... .................................................................. Fosfomycin 1 g Amoxicillin ....................................................................................... ................................................................... 1 g ..................................................................................... .................................................................. Fosfomycin 4 g Amoxicillin ....................................................................................... ................................................................... 2 g Gentamicin ...................................................................................... -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Murexal 10 mg/mL solution for injection in pre-filled syringe 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of solution for injection contains 10 mg of suxamethonium chloride anhydrous (as 11 mg of suxamethonium chloride dihydrate). Each 10 ml pre-filled syringe contains 100 mg of suxamethonium chloride anhydrous (as 110 mg of suxamethonium chloride dihydrate). Excipient with known effect: Each ml of solution for injection contains 2.79 mg equivalent to 0.12 mmol of sodium. Each 10 ml pre-filled syringe contains 27.9 mg equivalent to 1.2 mmol of sodium. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection (injection). Clear and colourless solution. pH: 3.0 – 4.5 Osmolality: 250-350 mOsm/Kg 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Murexal is indicated as a muscle relaxant to facilitate endotracheal intubation during induction of general anaesthesia or emergency situations, in adults and paediatric population above 12 years of age. 4.2 Posology and method of administration Suxamethonium should be administered only by or under close supervision of an experienced clinician (anaesthesist, intensivist, emergency physician) familiar with its action, characteristics and hazards, who is skilled in the management of intubation and artificial respiration and only where there are adequate facilities for immediate endotracheal intubation with administration of oxygen by intermittent positive pressure ventilation. It is given intravenously after anaesthesia has been induced and should not be administered to the conscious patient. Posology Adults To achieve endotracheal intubation, suxamethonium chloride is usually administered by bolus intravenous injection in a dose of 1 mg/kg body weight. -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. TRACRIUM (atracurium besilate 10 mg/mL injections (2.5 mL and 5.0 mL)) 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mL ampoule contains 25 mg atracurium besilate, each 5 mL ampoule contains 50 mg atracurium besilate. TRACRIUM 2.5 mL and 5.0 mL injections contain no preservative. 3. PHARMACEUTICAL FORM TRACRIUM injection is a clear, faintly yellow, sterile, aqueous solution in a glass ampoule containing 10 mg/mL atracurium besilate. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications TRACRIUM is a highly selective, competitive or non-depolarising neuromuscular blocking agent which is used as an adjunct to general anaesthesia to enable tracheal intubation to be performed and to relax skeletal muscles during surgery or controlled ventilation, and to facilitate mechanical ventilation in Intensive Care Unit (ICU) patients. 4.2 Dose and method of administration Use in adults Injection TRACRIUM is administered by intravenous injection. The dosage range for adults is 0.3 to 0.6 mg/kg (depending on the duration of full block required) and will provide adequate relaxation for 15 to 35 minutes. Endotracheal intubation can usually be accomplished within 90 seconds from the intravenous injection of 0.5 to 0.6 mg/kg. Full block can be prolonged with supplementary doses of 0.1 to 0.2 mg/kg as required. Successive supplementary dosing does not give rise to accumulation of neuromuscular blocking effect. Spontaneous recovery from the end of full block occurs in about 35 minutes as measured by the restoration of the tetanic response to 95% of normal neuromuscular function. -

World Health Organization Model List of Essential Medicines, 21St List, 2019
World Health Organizatio n Model List of Essential Medicines 21st List 2019 World Health Organizatio n Model List of Essential Medicines 21st List 2019 WHO/MVP/EMP/IAU/2019.06 © World Health Organization 2019 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. CIP data are available at http://apps.who.int/iris. -

Section 2.6.4 Pharmacokinetics Written Summary EMTRICITABINE
SECTION 2.6 NONCLINICAL SUMMARY Section 2.6.4 Pharmacokinetics Written Summary EMTRICITABINE/ RILPIVIRINE/ TENOFOVIR DISOPROXIL FUMARATE FIXED-DOSE COMBINATION 17 August 2010 CONFIDENTIAL AND PROPRIETARY INFORMATION Emtricitabine/Rilpivirine/Tenofovir Disoproxil Fumarate Section 2.6.4 Pharmacokinetics Written Summary Final TABLE OF CONTENTS SECTION 2.6 NONCLINICAL SUMMARY........................................................................................................1 TABLE OF CONTENTS .......................................................................................................................................2 GLOSSARY OF ABBREVIATIONS AND DEFINITION OF TERMS ..............................................................5 2.6. NONCLINICAL SUMMARY.......................................................................................................................8 2.6.4. PHARMACOKINETICS WRITTEN SUMMARY .........................................................................8 2.6.4.1. Brief Summary................................................................................................................8 2.6.4.2. Methods of Analysis .....................................................................................................14 2.6.4.2.1. Emtricitabine............................................................................................14 2.6.4.2.2. Rilpivirine ................................................................................................14 2.6.4.2.3. Tenofovir Disoproxil Fumarate