Comparative Study of Different Doses of Rocuronium Bromide For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
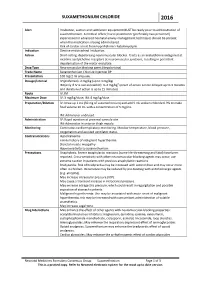
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

Vecuronium Bromide As Control
A COMPARATIVE STUDY TO FIND AN IDEAL INTUBATING DOSE OF INJ. ROCURONIUM BROMIDE USING INJ. VECURONIUM BROMIDE AS CONTROL. Dissertation submitted In partial fulfillment for the award of M.D DEGREE EXAMINATION M.D. ANAESTHESIOLOGY & CRITICAL CARE BRANCH X GOVT KILPAUK MEDICAL COLLEGE AND HOSPITAL SUBMITTED TO THE TAMILNADU DR. MGR MEDICAL UNIVERSITY CHENNAI APRIL – 2011 1 DECLARATION I, Dr. D.Shunmuga Priya, solemnly declare that the dissertation, “A COMPARATIVE STUDY TO FIND AN IDEAL INTUBATING DOSE OF INJ. ROCURONIUM BROMIDE USING INJ. VECURONIUM BROMIDE AS CONTROL” is a bonafide work done by me in the Department of Anaesthesiology and Critical Care, Government Kilpauk, Medical College, Chennai under the able guidance of Prof. Dr. P.S. Shanmugam, MD., DA., Professor and HOD, Department of Anaesthesiology and Critical Care, Government Kilpauk Medical College, Chennai. Place : Chennai Date : (Dr. D.SHUNMUGA PRIYA) 5 CERTIFICATE This is to certify that this dissertation titled “A COMPARATIVE STUDY TO FIND AN IDEAL INTUBATING DOSE OF INJ. ROCURONIUM BROMIDE USING INJ. VECURONIUM BROMIDE AS CONTROL” has been prepared by Dr. D.SHUNMUGA PRIYA under my supervision in the Department of Anaesthesiology and Critical Care, Government Kilpauk Medical College, Chennai during the academic period 2008-2011 and is being submitted to the Tamil Nadu Dr. M.G.R. Medical University, Chennai in partial fulfillment of the University regulation for the award of the Degree of Doctor of Medicine (MD Anaesthesiology and Critical Care) and her dissertation is a bonafide work. Prof..Dr. V.Kanagasabai, M.D Prof. Dr. P.S.Shanmugam, M.D., D.A. DEAN Professor & HOD, Government Kilpauk Medical College Department of Anaesthesiology and & Hospital, Critical Care, Chennai. -

Rocuronium Bromide PRODUCT DATA SHEET Issue Date 01/06/2020
Rocuronium Bromide PRODUCT DATA SHEET issue date 01/06/2020 Product Name: Rocuronium Bromide Product Number: R045 CAS Number: 119302-91-9 Molecular Formula: C32H53BrN2O4 Molecular Weight: 609.68 Form: Powder Appearance: Off-white to yellowish powder Solubility: Soluble in water, ethanol, and DMSO. Source: Synthetic Storage Conditions: ≤30°C Description: Rocuronium Bromide is the bromide salt form of Rocuronium, a fumarate, aminosteroid type non-depolarizing neuromuscular blocking agent that relaxes the skeletal muscle. It was introduced in 1994, and has a similar pharmacokinetic profile to Vecuronium, as it is a derivative of the 3-hydroxy metabolite of Vecuronium. It competes with acetylcholine in binding to cholinergic receptors at neuromuscular junctions. Rocuronium Bromide is soluble in water, ethanol, and DMSO. Mechanism of Action: Rocuronium Bromide competes with acetylcholine and binds to cholinergic receptors at neuromuscular junctions. It binds to nicotinic receptors in the neuromuscular junction. It is classified as a neuromuscular nondepolarizing agent since it does not cause depolarization of the motor end plate. References: Anzenbacherova et al (2015) Interaction of Rocuronium with human liver cytochromes P450. J. Pharmacol. Sci. 127(2):190-195 PMID 25727956 Cembala TM, Sherwin JD, Tidmarsh MD, Appadu BL and Lambert DG (1998) Interaction of neuromuscular blocking drugs with recombinant human m1-m5 muscarinic receptors expressed in Chinese hamster ovary cells. B. J. Pharmacol. 125(5):1088-1094 PMID 9846649 Koksal PM and Gürbüzel M(2015) Analysis of genotoxic activity of ketamine and Rocuronium Bromide using the somatic mutation and recombination test in Drosophila melanogaster. Environ Toxicol Pharmacol. 39(2):628-634 PMID 25682000 Sauer et al (2017) Rocuronium is more hepatotoxic than succinylcholine in vitro. -

COMPARISON of ROCURONIUM BROMIDE and SUCCINYLCHOLINE CHLORIDE for USE DURING RAPID SEQUENCE INTUBATION in ADULTS Ch
DOI: 10.18410/jebmh/2015/672 ORIGINAL ARTICLE COMPARISON OF ROCURONIUM BROMIDE AND SUCCINYLCHOLINE CHLORIDE FOR USE DURING RAPID SEQUENCE INTUBATION IN ADULTS Ch. Penchalaiah1, N. Jagadeesh Babu2, T. Kumar3 HOW TO CITE THIS ARTICLE: Ch. Penchalaiah, N. Jagadeesh Babu, T. Kumar. ”Comparison of Rocuronium Bromide and Succinylcholine Chloride for Use during Rapid Sequence Intubation in Adults”. Journal of Evidence based Medicine and Healthcare; Volume 2, Issue 32, August 10, 2015; Page: 4796-4806, DOI: 10.18410/jebmh/2015/672 ABSTRACT: BACKGROUND AND OBJECTIVE: The goal of rapid sequence intubation is to secure the patients airway smoothly and quickly, minimizing the chances of regurgitation and aspiration of gastric contents. Traditionally succinylcholine chloride has been the neuromuscular blocking drug of choice for use in rapid sequence intubation because of its rapid onset of action and profound relaxation. Succinylcholine chloride remains unsurpassed in providing ideal intubating conditions. However the use of succinylcholine chloride is associated with many side effects like muscle pain, bradycardia, hyperkalaemia and rise in intragastric and intraocular pressure. Rocuronium bromide is the only drug currently available which has the rapidity of onset of action like succinylcholine chloride. Hence the present study was undertaken to compare rocuronium bromide with succinylcholine chloride for use during rapid sequence intubation in adult patients. METHODOLOGY: The study population consisted of 90 patients aged between 18-60 years posted for various elective surgeries requiring general anaesthesia. Study population was randomly divided into 3 groups with 30 patients in each sub group. 1. Group I: Intubated with 1 mg kg-1 of succinylcholine chloride (n=30). -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

INTUBATING CONDITIONS and INJECTION PAIN - Cisatracurium Or Rocuronium Versus Rocuronium-Cisatracurium Combination
INTUBATING CONDITIONS AND INJECTION PAIN - Cisatracurium or Rocuronium versus Rocuronium-Cisatracurium Combination - * * AHED ZEIDAN ,NAZIH NAHLE , ** *** HILAL MAALIKI AND ANIS BARAKA Summary The present report evaluates the incidence of pain on intravenous injection and the condition of tracheal intubation at one minute following the administration of cisatracurium or rocuronium versus rocuronium- cisatracurium combination. We studied 60 patients, ASA 1, aged 18-60 years, undergoing elective surgical procedures. The patients were randomly assigned to 3 groups who received intravenously either 0.15 mg/kg cisatracurium [2ED95], 0,6 mg rocuronium [2ED95] or a combination of 0.075 mg/kg cisatracurium [1ED95], plus 0.3 mg rocuronium [1ED95]. In the awake patients, the pain on injection of muscle relaxant was assessed on a four point scale (none, mild, moderate, severe). Administration of the relaxant was followed by 1-2 mg/kg of lidocaine and 2 mg/kg propofol. Oro- tracheal intubation was performed 60 seconds following the administration of the relaxant. The intubating conditions were assessed and rated as excellent, good, fair or poor. * MD, Staff Anaesthesiologist, Department of Anaesthesiology, Sahel General Hospital, Beirut, Lebanon. **MD Staff Anaesthesiologist, Department of Anaesthesiology, Beirut Governmental University Hospital, Beirut, Lebanon. *** MD, FRCA, Professor and and Chairman, Department of Anesthesiology, American University of Beirut Medical Center, Beirut, Lebanon. Corresponding Author: Ahed Zeidan, Department of Anaesthesiology, Sahel General Hospital, Airport Ave. P.O. Box: 99/25-Ghobeiry, Beirut-Lebanon. Phone: 961-1-858333. Fax: 961-1-840146, E-mail: [email protected]. 879 M.E.J. ANESTH 18 (5), 2006 880 AHED ZEIDAN ET. AL The administration of 2ED95 cisatracurium resulted in poor intubating conditions at 60s, without pain on injection. -

Zambia Essential Medicine List (Zeml) 03 2013
ZAMBIA ESSENTIAL MEDICINE LIST (ZEML) 03 2013 Drug Presentation Level VEN 1 Drugs used in anaesthesia 1.1. Drugs used in general anaesthesia 1.1.1 Intravenous and intramuscular anaesthetics 1.1.1.1 Ketamin injection 10mg/ml (10 ml) II-IV V 1.1.1.2 Thiopentone sodium powder for reconstitution 1g and 5g vials II-IV V 1.1.2 Inhalation anaesthetics 1.1.2.1 Halothane inhalation II-IV V 1.1.2.2 Nitrous oxide medical gas II-IV E 1.1.3 Muscle relaxants 1.1.3.1 Suxamethonium chloride injection 50mg/ml, (2ml) II-IV V 1.1.4 Anticholinesterases 1.1.4.1 Neostigmine injection 2.5mg/ml, (1ml) II-IV V 1.2 Drugs used in local anaesthesia 1.2.1. Lignocaine injection 1% (10ml, 50ml) I-IV V 1.2.2 Lignocaine + adrenaline dental cartridge injection 2% (1 in 80,000) II-IV V 1.3 Drugs used in spinal anaesthesia 1.3.1 Bupivacaine/glucose injection 0.5%, (4ml) IV E 1 Drug Presentation Level VEN 2. Drugs acting on the gastrointestinal system 2.1 Antacids 2.1.1 Aluminium hydroxide gel, chewable tablets I-IV E 2.12 Magnesium trisilicate Compound chewable tablets, mixture I-IV E 2.2. Antispasmodics 2.2.1 Hyoscine butyl bromide injection 20mg/ml, (1ml) II-IV E 2.2.2 Propantheline bromide tablets 15mg I-IV E 2.3 Ulcer healing drugs 2.3.1 Cimetidine tablets 200mg II-IV E 2.3.2 Omeprazole tablets 10mg, II-IV E 2.3.3 Ranitidine tablets 150mg II-IV E 2.3.4 Tripotassium dicitratobismuthate tablets 120mg II-IV E 2.3.5 Clarithromycin tablets 250mg II-IV E 2.4. -

2018 Product List Expertise in Injectables
2018 Product list Expertise in injectables Flucloxacillin ................................................................................ ................................................... 250 mg Antibiotics ................................................................................ ................................................... Flucloxacillin 500 mg Amoxicillin ....................................................................................... .................................................... 250 mg ................................................................................ .................................................................. Flucloxacillin 1 g Amoxicillin ....................................................................................... .................................................... 500 mg ..................................................................................... .................................................................. Fosfomycin 1 g Amoxicillin ....................................................................................... ................................................................... 1 g ..................................................................................... .................................................................. Fosfomycin 4 g Amoxicillin ....................................................................................... ................................................................... 2 g Gentamicin ...................................................................................... -

Reversal of Neuromuscular Bl.Pdf
JosephJoseph F.F. Answine,Answine, MDMD Staff Anesthesiologist Pinnacle Health Hospitals Harrisburg, PA Clinical Associate Professor of Anesthesiology Pennsylvania State University Hospital ReversalReversal ofof NeuromuscularNeuromuscular BlockadeBlockade DefiniDefinitionstions z ED95 - dose required to produce 95% suppression of the first twitch response. z 2xED95 – the ED95 multiplied by 2 / commonly used as the standard intubating dose for a NMBA. z T1 and T4 – first and fourth twitch heights (usually given as a % of the original twitch height). z Onset Time – end of injection of the NMBA to 95% T1 suppression. z Recovery Time – time from induction to 25% recovery of T1 (NMBAs are readily reversed with acetylcholinesterase inhibitors at this point). z Recovery Index – time from 25% to 75% T1. PharmacokineticsPharmacokinetics andand PharmacodynamicsPharmacodynamics z What is Pharmacokinetics? z The process by which a drug is absorbed, distributed, metabolized and eliminated by the body. z What is Pharmacodynamics? z The study of the action or effects of a drug on living organisms. Or, it is the study of the biochemical and physiological effects of drugs. For example; rocuronium reversibly binds to the post synaptic endplate, thereby, inhibiting the binding of acetylcholine. StructuralStructural ClassesClasses ofof NondepolarizingNondepolarizing RelaxantsRelaxants z Steroids: rocuronium bromide, vecuronium bromide, pancuronium bromide, pipecuronium bromide. z Benzylisoquinoliniums: atracurium besylate, mivacurium chloride, doxacurium chloride, cisatracurium besylate z Isoquinolones: curare, metocurine OnsetOnset ofof paralysisparalysis isis affectedaffected by:by: z Dose (relative to ED95) z Potency (number of molecules) z KEO (plasma equilibrium constant - chemistry/blood flow) — determined by factors that modify access to the neuromuscular junction such as cardiac output, distance of the muscle from the heart, and muscle blood flow (pharmacokinetic variables). -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Murexal 10 mg/mL solution for injection in pre-filled syringe 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of solution for injection contains 10 mg of suxamethonium chloride anhydrous (as 11 mg of suxamethonium chloride dihydrate). Each 10 ml pre-filled syringe contains 100 mg of suxamethonium chloride anhydrous (as 110 mg of suxamethonium chloride dihydrate). Excipient with known effect: Each ml of solution for injection contains 2.79 mg equivalent to 0.12 mmol of sodium. Each 10 ml pre-filled syringe contains 27.9 mg equivalent to 1.2 mmol of sodium. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution for injection (injection). Clear and colourless solution. pH: 3.0 – 4.5 Osmolality: 250-350 mOsm/Kg 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Murexal is indicated as a muscle relaxant to facilitate endotracheal intubation during induction of general anaesthesia or emergency situations, in adults and paediatric population above 12 years of age. 4.2 Posology and method of administration Suxamethonium should be administered only by or under close supervision of an experienced clinician (anaesthesist, intensivist, emergency physician) familiar with its action, characteristics and hazards, who is skilled in the management of intubation and artificial respiration and only where there are adequate facilities for immediate endotracheal intubation with administration of oxygen by intermittent positive pressure ventilation. It is given intravenously after anaesthesia has been induced and should not be administered to the conscious patient. Posology Adults To achieve endotracheal intubation, suxamethonium chloride is usually administered by bolus intravenous injection in a dose of 1 mg/kg body weight. -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. TRACRIUM (atracurium besilate 10 mg/mL injections (2.5 mL and 5.0 mL)) 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mL ampoule contains 25 mg atracurium besilate, each 5 mL ampoule contains 50 mg atracurium besilate. TRACRIUM 2.5 mL and 5.0 mL injections contain no preservative. 3. PHARMACEUTICAL FORM TRACRIUM injection is a clear, faintly yellow, sterile, aqueous solution in a glass ampoule containing 10 mg/mL atracurium besilate. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications TRACRIUM is a highly selective, competitive or non-depolarising neuromuscular blocking agent which is used as an adjunct to general anaesthesia to enable tracheal intubation to be performed and to relax skeletal muscles during surgery or controlled ventilation, and to facilitate mechanical ventilation in Intensive Care Unit (ICU) patients. 4.2 Dose and method of administration Use in adults Injection TRACRIUM is administered by intravenous injection. The dosage range for adults is 0.3 to 0.6 mg/kg (depending on the duration of full block required) and will provide adequate relaxation for 15 to 35 minutes. Endotracheal intubation can usually be accomplished within 90 seconds from the intravenous injection of 0.5 to 0.6 mg/kg. Full block can be prolonged with supplementary doses of 0.1 to 0.2 mg/kg as required. Successive supplementary dosing does not give rise to accumulation of neuromuscular blocking effect. Spontaneous recovery from the end of full block occurs in about 35 minutes as measured by the restoration of the tetanic response to 95% of normal neuromuscular function. -

Dblrocuroniuminj.Pdf
NEW ZEALAND DATA SHEET 1. PRODUCT NAME DBLTM Rocuronium Bromide Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of DBLTM Rocuronium Bromide injection contains 10 mg rocuronium bromide and the following inactive ingredients sodium acetate, sodium chloride and acetic acid and/or sodium hydroxide q.s. to adjust pH to within the range of 3.8 -4.2. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection Rocuronium bromide is an off-white to pale-yellow or slightly pink amorphous powder. Rocuronium bromide is readily soluble in water (> 200 mg/mL) and a 1% w/v solution in water has a pH of 8.9 – 9.5. In aqueous solution rocuronium bromide is more stable at acidic pH. DBLTM Rocuronium Bromide Injection is supplied as a sterile, nonpyrogenic, isotonic solution that is clear, colourless to faintly yellow administered by intravenous bolus or infusion. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications DBLTM Rocuronium Bromide Injection is indicated to provide skeletal muscle relaxation during surgery. DBLTM Rocuronium Bromide Injection is also indicated as an adjunct to general anaesthesia to facilitate tracheal intubation during routine induction, and during rapid sequence induction when suxamethonium is contraindicated. DBLTM Rocuronium Bromide Injection is also indicated as an adjunct in the Intensive Care Unit (ICU) to facilitate intubation and mechanical ventilation. 4.2 Dose and method of administration Dose Like other neuromuscular blocking agents DBLTM Rocuronium Bromide Injection should only be administered by, or under supervision of, experienced clinicians who are familiar with the action and use of these agents.