Acetylcholinesterase: the “Hub” for Neurodegenerative Diseases And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

Carbamate Pesticides Aldicarb Aldicarb Sulfoxide Aldicarb Sulfone
Connecticut General Statutes Sec 19a-29a requires the Commissioner of Public Health to annually publish a list setting forth all analytes and matrices for which certification for testing is required. Connecticut ELCP Drinking Water Analytes Revised 05/31/2018 Microbiology Total Coliforms Fecal Coliforms/ E. Coli Carbamate Pesticides Legionella Aldicarb Cryptosporidium Aldicarb Sulfoxide Giardia Aldicarb Sulfone Carbaryl Physicals Carbofuran Turbidity 3-Hydroxycarbofuran pH Methomyl Conductivity Oxamyl (Vydate) Minerals Chlorinated Herbicides Alkalinity, as CaCO3 2,4-D Bromide Dalapon Chloride Dicamba Chlorine, free residual Dinoseb Chlorine, total residual Endothall Fluoride Picloram Hardness, Calcium as Pentachlorophenol CaCO3 Hardness, Total as CaCO3 Silica Chlorinated Pesticides/PCB's Sulfate Aldrin Chlordane (Technical) Nutrients Dieldrin Endrin Ammonia Heptachlor Nitrate Heptachlor Epoxide Nitrite Lindane (gamma-BHC) o-Phosphate Metolachlor Total Phosphorus Methoxychlor PCB's (individual aroclors) Note 1 PCB's (as decachlorobiphenyl) Note 1 Demands Toxaphene TOC Nitrogen-Phosphorus Compounds Alachlor Metals Atrazine Aluminum Butachlor Antimony Diquat Arsenic Glyphosate Barium Metribuzin Beryllium Paraquat Boron Propachlor Cadmium Simazine Calcium Chromium Copper SVOC's Iron Benzo(a)pyrene Lead bis-(2-ethylhexyl)phthalate Magnesium bis-(ethylhexyl)adipate Manganese Hexachlorobenzene Mercury Hexachlorocyclopentadiene Molybdenum Nickel Potassium Miscellaneous Organics Selenium Dibromochloropropane (DBCP) Silver Ethylene Dibromide (EDB) -

Monocrotophos Proposed AEGL Document
MONOCROTOPHOS Page 1 of 32 Proposed 10/2009 v.1 1 2 3 4 ACUTE EXPOSURE GUIDELINE LEVELS 5 (AEGLs) 6 7 8 PROPOSED 9 10 11 12 13 MONOCROTOPHOS 14 (CAS Reg. No. 6923-22-4) 15 16 17 18 19 20 MONOCROTOPHOS Page 2 of 32 Proposed 10/2009 v.1 1 2 PREFACE 3 4 Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 5 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous 6 Substances (NAC/AEGL Committee) has been established to identify, review and interpret 7 relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic 8 chemicals. 9 10 AEGLs represent threshold exposure limits for the general public and are applicable to 11 emergency exposure periods ranging from 10 minutes to 8 hours. Three levels C AEGL-1, 12 AEGL-2 and AEGL-3 C are developed for each of five exposure periods (10 and 30 minutes, 1 13 hour, 4 hours, and 8 hours) and are distinguished by varying degree of severity of toxic effects. 14 The three AEGLs are defined as follows: 15 16 AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per 17 cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general 18 population, including susceptible individuals, could experience notable discomfort, irritation, or 19 certain asymptomatic, non-sensory effects. However, the effects are not disabling and are 20 transient and reversible upon cessation of exposure. -

Galantamine Potentiates the Neuroprotective Effect of Memantine Against NMDA-Induced Excitotoxicity Joao~ P
Galantamine potentiates the neuroprotective effect of memantine against NMDA-induced excitotoxicity Joao~ P. Lopes1, Glauco Tarozzo1, Angelo Reggiani1, Daniele Piomelli1,2 & Andrea Cavalli1,3 1D3 – Drug Discovery and Development Department, Istituto Italiano di Tecnologia, Via Morego, 16163, Genova, Italy 2Departments of Anatomy and Neurobiology and Biological Chemistry, University of California, Irvine, CA, 92697-4621 3Department of Pharmacy and Biotechnologies, Alma Mater Studiorum, Bologna University, Via Belmeloro, 40126, Bologna, Italy Keywords Abstract Alzheimer’s disease, drug combination, N NMDA neurotoxicity, NR2B, The combination of memantine, an -methyl-D-aspartate (NMDA) receptor polypharmacology, primary cortical neurons antagonist, with an acetylcholinesterase inhibitor (AChEI) is the current stan- dard of care in Alzheimer’s disease (AD). Galantamine, an AChEI currently Correspondence marketed for the treatment of AD, exerts memory-enhancing and neuroprotec- Andrea Cavalli, D3 – Drug Discovery and tive effects via activation of nicotinic acetylcholine receptors (nAChRs). Here, Development Department, Istituto Italiano we investigated the neuroprotective properties of galantamine in primary cul- di Tecnologia – Via Morego, 30, 16163 tures of rat cortical neurons when given alone or in combination with meman- Genova, Italy. Tel: +39 010 71781530; Fax: +39 010 tine. In agreement with previous findings, we found that memantine was fully 71781228; E-mail: [email protected] effective in reversing NMDA toxicity at concentrations of 2.5 and 5 lmol/L. Galantamine also completely reversed NMDA toxicity at a concentration of Funding Information 5 lmol/L. The a7 and a4b2 nAChR antagonists, methyllycaconitine, and dihy- No funding information provided. dro-b-erythroidine blocked the neuroprotective effect of galantamine, demon- strating the involvement of nAChRs. -

Downloads/Pgm Hydrus1d/HYDRUS-4.16.Pdf (Accessed on 20 April 2019)
water Article Assessment of the Environmental Risk of Pesticides Leaching at the Watershed Scale under Arid Climatic Conditions and Low Recharge Rates Hesham M. Ibrahim 1,2,* and Ali M. Al-Turki 1 1 Department of Soil Science, College of Food and Agricultural Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia; [email protected] 2 Department of Soils and Water, Faculty of Agriculture, Suez Canal University, Ismailia 41522, Egypt * Correspondence: [email protected]; Tel.: +966-501728656 Received: 5 January 2020; Accepted: 2 February 2020; Published: 5 February 2020 Abstract: The assessment of the vulnerability of soil and groundwater resources to pesticide contamination is important to reduce the risk of environmental pollution. The applicability of the expanded attenuation factor (EAF) to assess leaching potential of 30 pesticides was investigated under 1 four recharge rates (0.0003–0.002 m d− ) in the arid environment of the Jazan watershed. EAF results revealed that Picloram, Carbofuran, Monocrotophos, and 2,4-D pesticides showed high leaching potential, mainly because of their low KOC, and relatively longer t1/2. In addition, medium leaching potential was observed with six more pesticides (Atrazine, Aldicarb, Simazine, Methomyl, Oxamyl, and Lindane). Regardless of the recharge rate, all other pesticides showed a very low leaching potential in the Jazan watershed. Sensitivity analysis revealed that the output of the EAF index is most sensitive to the fraction of organic carbon ( foc), water content at field capacity (θFC ), recharge rate (q), and partition coefficient (KOC), and least sensitive to soil bulk density (ρb) and air-filled porosity (na). -

Chemical Warfare Agent (CWA) Identification Overview
Physicians for Human Rights Chemical Warfare Agent (CWA) Identification Overview Chemical Warfare Agent Identification Fact Sheet Series Table of Contents This Chemical Warfare Agent (CWA) Identification Fact Sheet is part 2 Physical Properties of a Physicians for Human Rights (PHR) series designed to fill a gap in 2 VX (Nerve Agent) 2 Sarin (Nerve Agent) knowledge among medical first responders to possible CWA attacks. 2 Tabun (Nerve Agent) This document in particular outlines differences between a select 2 BZ (Incapacitating Agent) group of vesicants and nerve agents, the deployment of which would 2 Mustard Gas (Vesicant) necessitate emergency medical treatment and documentation. 3 Collecting Samples to Test for Exposure 4 Protection PHR hopes that, by referencing these fact sheets, medical professionals 5 Symptoms may be able to correctly diagnose, treat, and document evidence of 6 Differential Diagnosis exposure to CWAs. Information in this fact sheet has been compiled from 8 Decontimanation 9 Treatment publicly available sources. 9 Abbreviations A series of detailed CWA fact sheets outlining in detail those properties and treatment regimes unique to each CWA is available at physiciansforhumanrights.org/training/chemical-weapons. phr.org Chemical Warfare Agent (CWA) Identification Overview 1 Collect urine samples, and blood and hair samples if possible, immediately after exposure Physical Properties VX • A lethal dose (10 mg) of VX, absorbed through the skin, can kill within minutes (Nerve Agent) • Can remain in environment for weeks -

Reference List of Drugs with Potential Anticholinergic Effects 1, 2, 3, 4, 5
ANTICHOLINERGICS: Reference List of Drugs with Potential Anticholinergic Effects 1, 2, 3, 4, 5 J Bareham BSP © www.RxFiles.ca Aug 2021 WHENEVER POSSIBLE, AVOID DRUGS WITH MODERATE TO HIGH ANTICHOLINERGIC ACTIVITY IN OLDER ADULTS (>65 YEARS OF AGE) Low Anticholinergic Activity; Moderate/High Anticholinergic Activity -B in combo Beers Antibiotics Antiparkinsonian Cardiovascular Agents Immunosuppressants ampicillin *ALL AVAILABLE AS amantadine SYMMETREL atenolol TENORMIN azaTHIOprine IMURAN cefOXitin GENERIC benztropine mesylate COGENTIN captopril CAPOTEN cyclosporine NEORAL clindamycin bromocriptine PARLODEL chlorthalidone GENERIC ONLY hydrocortisone CORTEF gentamicin (Oint & Sol’n NIHB covered) carbidopa/levodopa SINEMET digoxin LANOXIN, TOLOXIN methylprednisolone MEDROL piperacillin entacapone COMTAN dilTIAZem CARDIZEM, TIAZAC prednisone WINPRED dipyridamole PERSANTINE, ethopropazine PARSITAN vancomycin phenelzine NARDIL AGGRENOX disopyramide RYTHMODAN Muscle Relaxants pramipexole MIRAPEX Antidepressants baclofen LIORESAL ( on intrathecal only) procyclidine KEMADRIN furosemide LASIX amitriptyline ELAVIL cyclobenzaprine FLEXERIL selegiline ELDEPRYL hydrALAZINE APRESOLINE clomiPRAMINE ANAFRANIL isosorbide ISORDIL methocarbamol ROBAXIN OTC trihexyphenidyl ARTANE desipramine NORPRAMIN metoprolol LOPRESOR orphenadrine NORFLEX OTC doxepin >6mg SINEQUAN Antipsychotics NIFEdipine ADALAT tiZANidine ZANAFLEX A imipramine TOFRANIL quiNIDine GENERIC ONLY C ARIPiprazole ABILIFY & MAINTENA -

Florida State Emergency Response Commission
Florida State Emergency Response Commission Sub-Committee on Training (SOT) HAZARDOUS MATERIALS MEDICAL TREATMENT PROTOCOLS Version 3.3 TOXIDROMES Toxidromes are clinical syndromes that the patient presents with. These patterns of signs and symptoms are essential for the successful recognition of chemical exposure. The toxidromes identified in this protocol are chemical exposure based while others such as the opioids are found within general medical protocol. These chemical toxidromes are identified clinically into five syndromes: Irritant Gas Toxidrome Asphyxiant Toxidrome Corrosive Toxidrome Hydrocarbon and Halogenated Hydrocarbons Toxidrome Cholinergic Toxidrome Each can present as a clinical manifestation of the chemical/poisoning involved with some cross-over between toxidromes. This list combines the toxic syndromes found within NFPA 473 (A.5.4.1(2) and traditional syndromes. Toxidrome Correlation to NFPA Standard 473 and Traditional Syndromes Toxidrome NFPA 473 A.5.4.1(2) Hazardous Materials Protocol Correlation Irritant Gas (j) Irritants Bronchospasm OC Pepper spray & lacrimants Asphyxiant (c) Chemical asphyxiants Carbon Monoxide (d) Simple asphyxiants Aniline dyes, Nitriles, Nitrares (h) Blood Agents Cyanide & Hydrogen Sulfide (n) Nitrogen Compounds Closed Space Fires Simple Asphyxants Corrosive (a) Corrosives Hydrofluroic Acid (g) Vesicants Chemical burns to the eye Choramine and Chlorine Hydrocarbon (e) Organic solvents Phenol and (q) Phenolic Compounds Halogenated Hydrocarbons Halogenated Hydrocarbons Cholinergic (b) Pesticides -

Chlorine.Pdf
Chlorine 7782-50-5 Hazard Summary Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. Chronic (long-term) exposure to chlorine gas in workers has resulted in respiratory effects, including eye and throat irritation and airflow obstruction. No information is available on the carcinogenic effects of chlorine in humans from inhalation exposure. A National Toxicology Program (NTP) study showed no evidence of carcinogenic activity in male rats or male and female mice, and equivocal evidence in female rats, from ingestion of chlorinated water. EPA has not classified chlorine for potential carcinogenicity. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (2), which contains information on oral chronic toxicity and the RfD, The California Environmental Protection Agency's (CalEPA's) Technical Support Document for the Determination of Noncancer Chronic Reference Exposure Levels (3), and EPA's Drinking Water Criteria Document for Chlorine, Hypochlorous Acid and Hypochlorite Ion (1). Uses Chlorine is a commonly used household cleaner and disinfectant. It is widely used as an oxidizing agent in water treatment and chemical processes. It is also used in the bleaching process of wood pulp in pulp mills. (8) Sources and Potential Exposure Workers may be exposed to chlorine in industries where it is produced or used, particularly in the food and paper industries. In addition, persons breathing air around these industries may be exposed to chlorine. (1) Exposure to chlorine may also occur through drinking water and swimming pool water, where it is used as a disinfectant. -

Relief of Hypersensitivity After Nerve Injury from Systemic Donepezil
Saranya devi Relief of Hypersensitivity after Nerve Injury from ALN Systemic Donepezil Involves Spinal Cholinergic and γ-Aminobutyric Acid Mechanisms Spinal ACh and GABA Mechanisms for Donepezil Analgesia Masafumi Kimura, M.D.,* Ken-ichiro Hayashida, D.V.M., Ph.D.,† James C. Eisenach, M.D.,‡ KIMURA ET AL. Shigeru Saito M.D.,§ Hideaki Obata, M.D.‖ XX ABSTRACT What We Already Know about This Topic • Donepezil increases central nervous system acetylcholine lev- Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/118/1/173/259568/0000542-201301000-00030.pdf by guest on 28 September 2021 10.1097/ALN.0b013e318277a81c Background: Evoking spinal release of acetylcholine (ACh) els and is used for treatment of dementia produces antinociception in normal animals and reduces • Increases in spinal acetylcholine levels have been implicated in the relief of neuropathic pain hypersensitivity after nerve injury, and some studies suggest January that ACh-mediated analgesia relies on γ-aminobutyric acid (GABA)-ergic signaling in the spinal cord. In this study, the authors tested the spinal mechanisms underlying the anti- What This Article Tells Us That Is New 118 hypersensitivity effects of donepezil, a central nervous sys- • Donepezil increased spinal acetylcholine levels and γ- tem–penetrating cholinesterase inhibitor, in a rat model of aminobutyric acid levels to reduce nociceptive responses after nerve injury and represents a potential therapeutic pathway to neuropathic pain. reduce pain after nerve injury Methods: Male Sprague-Dawley rats were anesthetized, and L5 spinal nerve ligation was performed unilaterally. With- A antagonist; and CGP 35348 (30 μg), a γ-aminobutyric drawal threshold to a paw pressure test was measured before acid receptor type B antagonist. -
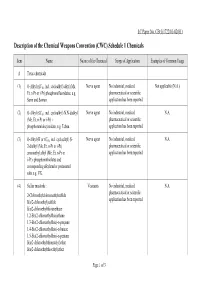
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention.