Case Report Carbamazepine Toxicity Following Oxybutynin And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skeletal Muscle Relaxants Review 12/17/2008
Skeletal Muscle Relaxants Review 12/17/2008 Copyright © 2007 - 2008 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 5181 Natorp Blvd., Suite 205 Mason, Ohio 45040 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to -

Drug Class Review on Skeletal Muscle Relaxants
Drug Class Review on Skeletal Muscle Relaxants Final Report Update 2 May 2005 Original Report Date: April 2003 Update 1 Report Date: January 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Roger Chou, MD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2005 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS Introduction........................................................................................................................4 Scope and -

Anaesthetic Implications of Calcium Channel Blockers
436 Anaesthetic implications of calcium channel Leonard C. Jenkins aA MD CM FRCPC blockers Peter J. Scoates a sc MD FRCPC CONTENTS The object of this review is to emphasize the anaesthetic implications of calcium channel block- Physiology - calcium/calcium channel blockers Uses of calcium channel blockers ers for the practising anaesthetist. These drugs have Traditional played an expanding role in therapeutics since their Angina pectoris introduction and thus anaesthetists can expect to see Arrhythmias increasing numbers of patients presenting for anaes- Hypertension thesia who are being treated with calcium channel Newer and investigational Cardiac blockers. Other reviews have emphasized the basic - Hypertrophic cardiomyopathy pharmacology of calcium channel blockers. 1-7 - Cold cardioplegia - Pulmonary hypertension Physiology - calcium/calcium channel blockers Actions on platelets Calcium plays an important role in many physio- Asthma Obstetrics logical processes, such as blood coagulation, en- - Premature labor zyme systems, muscle contraction, bone metabo- - Pre-eclampsia lism, synaptic transmission, and cell membrane Achalasia and oesophageal spasm excitability. Especially important is the role of Increased intraocular pressure therapy calcium in myocardial contractility and conduction Protective effect on kidney after radiocontrast Cerebral vasospasm as well as in vascular smooth muscle reactivity. 7 Induced hypotensive anaesthesia Thus, it can be anticipated that any drug interfering Drag interactions with calcium channel blockers with the action of calcium could have widespread With anaesthetic agents effects. Inhalation agents In order to understand the importance of calcium - Effect on haemodynamics - Effect on MAC in cellular excitation, it is necessary to review some Neuromuscular blockers membrane physiology. Cell membranes are pri- Effects on epinephrine-induced arrhythmias marily phospholipids arranged in a bilayer. -

Cyclobenzaprine and Back Pain: a Meta-Analysis
ORIGINAL INVESTIGATION Cyclobenzaprine and Back Pain A Meta-analysis Robert Browning, MD; Jeffrey L. Jackson, MD, MPH; Patrick G. O’Malley, MD, MPH Background: Back pain is a common problem for which 14 as were those treated with placebo. Slightly fewer than cyclobenzaprine hydrochloride is frequently pre- 3 individuals (2.7; 95% confidence interval, 2.0-4.2) scribed. needed treatment for 1 to improve. The magnitude of this improvement was modest, with an effect size of 0.38 to Objective: To perform a systematic review of cyclo- 0.58 in all 5 outcomes (local pain, muscle spasm, ten- benzaprine’s effectiveness in the treatment of back pain. derness to palpation, range of motion, and activities of daily living). Treatment efficacy for these 5 outcomes was Methods: We searched MEDLINE, PsycLIT, CINAHL, greatest early, in the first few days of treatment, declin- EMBASE, AIDSLINE, HEALTHSTAR, CANCERLIT, the ing after the first week. Patients receiving cyclobenzap- Cochrane Library, Micromedex, Federal Research in rine also experienced more adverse effects, the most com- Progress, and the references of reviewed articles, and con- mon being drowsiness. tacted Merck, Sharpe and Dohme for English-language, randomized, placebo-controlled trials of cyclobenzap- Conclusions: Cyclobenzaprine is more effective than rine in adults with back pain. Outcomes included global placebo in the management of back pain; the effect is improvement and 5 specific domains of back pain (local modest and comes at the price of greater adverse effects. pain, muscle spasm, range of motion, tenderness to pal- The effect is greatest in the first 4 days of treatment, sug- pation, and activities of daily living). -

Lorzone (Chlorzoxazone) Dose, Indications, Adverse Effects, Interactions... from PDR.Net 19/7/17 0:18
Lorzone (chlorzoxazone) dose, indications, adverse effects, interactions... from PDR.net 19/7/17 0:18 print Close window CLASSES Muscle Relaxants, Centrally Acting, Plain DEA CLASS Rx DESCRIPTION Oral, centrally acting, skeletal muscle relaxant; use has fallen out of favor; drug has been associated with hepatotoxicity. COMMON BRAND NAMES Lorzone, Parafon Forte DSC, Relax-DS HOW SUPPLIED Chlorzoxazone/Lorzone/Parafon Forte DSC/Relax-DS Oral Tab: 375mg, 500mg, 750mg DOSAGE & INDICATIONS For adjunctive therapy to rest, physical therapy, and other measures for the relief of musculoskeletal pain associated with acute, painful musculoskeletal conditions. Oral dosage Adults, Adolescents, and the Geriatric Initially, 250 to 500 mg PO given 3 to 4 times per day. Doses up to 750 mg PO given 3 to 4 times per day may be given for severe muscle spasm, but reduce when possible to lowest effective dose. Use with extreme caution in geriatric patients, as unpredictable, fatal hepatotoxicity has been reported. Children† Initially, 20 mg/kg/day PO given in 3 to 4 divided doses; or 600 mg/m2/day PO given in 3 to 4 divided doses. MAXIMUM DOSAGE Adults 3000 mg/day PO. Elderly 3000 mg/day PO. Adolescents 3000 mg/day PO. Children Maximum dosage limits are not available. DOSING CONSIDERATIONS Hepatic Impairment Avoid use in patients with hepatic impairment. Although causal factors are not known, chlorzoxazone has been associated with severe (and fatal) hepatotoxicity, even in patients without concurrent hepatic impairment. Renal Impairment No dosage adjustment is recommended; however, use with caution as metabolites are excreted via the kidneys. ADMINISTRATION Oral Administration Oral Solid Formulations If stomach upset occurs, chlorzoxazone may be taken with food or milk. -

Skeletal Muscle Relaxants Review 05/31/2010
Skeletal Muscle Relaxants Review 05/31/2010 Copyright © 2007 – 2010 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C 10101 Alliance Road, Suite 201 Cincinnati, Ohio 45242 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended -

The Effect of Dantrolene Sodium in Relation to Blood Levels in Spastic Patients After Prolonged Administration
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.44.4.334 on 1 April 1981. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1981, 44, 334-339 The effect of dantrolene sodium in relation to blood levels in spastic patients after prolonged administration W J MEYLER,* H BAKKER,t J J KOK,+ S AGOSTON,* AND H WESSELING* From the Institute of Clinical Pharmacology, State University Groningen, Groningen,* the Rehabilitation Hospital "De Hoogstraat," Leersum,t and the Rehabilitation Hospital, "Heliomare," Wijk aan Zee,+ The Netherlands SUMMARY In 25 patients with spasticity, pharmacokinetics and effects of dantrolene sodium were investigated after prolonged administration. A beneficial effect occurred in seven patients. The results were better on 100 mg daily than on a higher daily dose. An increase of the daily dose from 200 to 400 mg was not associated with higher blood levels. Many side effects were noted such as: anorexia, nausea, drowsiness, depression and muscle weakness. From this study we conclude that dantrolene sodium is a muscle relaxant with a weak to moderate effect in patients with spasticity; the effect at doses higher than 200 mg daily is probably poor. Protected by copyright. Dantrolene sodium is a muscle relaxant used in the mg), was supplied according to the following dosage treatment of spasticity. 1-9 Though effective in various schedule: 25 mg twice daily, 25 mg four times daily, 50 mg spastic conditions, reports on its therapeutic useful- four times daily, 100 mg four times daily, until the "opti- ness are conflicting and overshadowed by possible mal" dose of the drug was achieved, provided the maximum daily dose of 400 mg was not exceeded. -
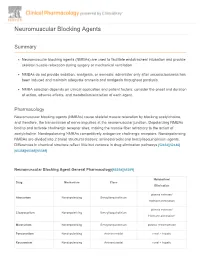
Neuromuscular Blocking Agents
Neuromuscular Blocking Agents Summary Neuromuscular blocking agents (NMBAs) are used to facilitate endotracheal intubation and provide skeletal muscle relaxation during surgery or mechanical ventilation. NMBAs do not provide sedation, analgesia, or amnesia; administer only after unconsciousness has been induced and maintain adequate amnesia and analgesia throughout paralysis. NMBA selection depends on clinical application and patient factors; consider the onset and duration of action, adverse effects, and metabolism/excretion of each agent. Pharmacology Neuromuscular blocking agents (NMBAs) cause skeletal muscle relaxation by blocking acetylcholine, and therefore, the transmission of nerve impulses at the neuromuscular junction. Depolarizing NMBAs bind to and activate cholinergic receptor sites, making the muscle fiber refractory to the action of acetylcholine. Nondepolarizing NMBAs competitively antagonize cholinergic receptors. Nondepolarizing NMBAs are divided into 2 broad structural classes: aminosteroidal and benzylisoquinolinium agents. Differences in chemical structure reflect little but variance in drug elimination pathways.[52452][52486] [65358][65369][65389] Neuromuscular Blocking Agent General Pharmacology[65358][65369] Metabolism/ Drug Mechanism Class Elimination plasma esterase/ Atracurium Nondepolarizing Benzylisoquinolinium Hofmann elimination plasma esterase/ Cisatracurium Nondepolarizing Benzylisoquinolinium Hofmann elimination* Mivacurium Nondepolarizing Benzylisoquinolinium plasma cholinesterase Pancuronium Nondepolarizing -

Cardiac Implications of Amlodipine-Dantrolene Combinations
50 Cardiac implications of amlodipine-dantrolene M. Freysz MD PhD,* Q. Timour PhO,~ C. Bernaud MD,:I: combinations L. Bertrix MD PhD,t G. Faucon MD PhD']" Purpose: Cardiac disorders, cardiac arrest and ventricular 0.001) and mean blood pressure from 79 • 4 to 70 • 3 mmHg `fibrillation in the most severe cases, have been observed after ( P < 0.01) by amlodipine, but dantrolene did not enhance and the administration of dantrolene to patients treated by vera- even counteracted these effects. Finally, potassium plasma pamil for coronary artery disease. This study was designed to concentration did not increase above 5.1 • 0.2 mmol.L -t examine the interaction of dantrolene with amlodipine, a dihy- under the dual influence of amlodipine and dantrolene. dropyridine. Conclusion: In usual clinical doses, dantrolene may be safely Methods: In 12 anaesthetized, open-chest pigs, the effects of administered concurrently with amlodipine. the interaction have been studied on heart rate, atrioventricu- lar conduction, monophasic action potential duration, intra- Objectifs: Des troubles cardiaques, arrEt cardiaque et fibrilla- ventricular conduction time, left ventricular dP/dt max and tion ventriculaire dans les cas les plus graves, ont dtd observes mean blood pressure. The study was performed with normal apr~s administration de dantrol~ne fi des patients traitds par coronary circulation and ischaemia of a large area of the left le vErapamil pour coronarite. Les interactions entre le dan- ventricule, obtained by complete occlusion of the left anterior trolbne et une dihydropyridine, l'amlodipine, font l'objet de descending coronary artery near its origin, under pacing at a cette Etude. -

Rapid Sequence Intubation
Challenges and Advances in Intubation: Rapid Sequence Intubation a,b,c,d, Sharon Elizabeth Mace, MD, FACEP, FAAP * KEYWORDS Intubation Rapid sequence intubation Endotracheal intubation DEFINITION/OVERVIEW Rapid sequence intubation (RSI) is a process whereby pharmacologic agents, specif- ically a sedative (eg, induction agent) and a neuromuscular blocking agent are admin- istered in rapid succession to facilitate endotracheal intubation.1 RSI in the emergency department (ED) usually is conducted under less than optimal conditions and should be differentiated from rapid sequence induction (also often ab- breviated RSI) as practiced by anesthesiologists in a more controlled environment in the operating room to induce anesthesia in patients requiring intubation.2–6 RSI used to secure a definitive airway in the ED frequently involves uncooperative, nonfasted, unstable, critically ill patients. In anesthesia, the goal of rapid sequence induction is to induce anesthesia while using a rapid sequence approach to decrease the possibil- ity of aspiration. With emergency RSI, the goal is to facilitate intubation with the addi- tional benefit of decreasing the risk of aspiration. Although there are no randomized, controlled trials documenting the benefits of RSI,7 and there is controversy regarding various steps in RSI in adult and pediatric pa- tients,8–13 RSI has become standard of care in emergency medicine airway manage- ment14–17 and has been advocated in the airway management of intensive care unit or critically ill patients.18 RSI has also -

Dantrolene Sodium/Methocarbamol 1897
Dantrolene Sodium/Methocarbamol 1897 3. Krause T, et al. Dantrolene—a review of its pharmacology, ther- Idrocilamide (rINN) Preparations apeutic use and new developments. Anaesthesia 2004; 59: 364–73. Idrocilamida; Idrocilamidum; LCB-29. N-(2-Hydroxyethyl)cin- Proprietary Preparations (details are given in Part 3) 4. Wedel DJ, et al. Clinical effects of intravenously administered namamide. Cz.: Dimexol; Dorsiflex; Neth.: Dorsiflex; Turk.: Dorsiflex. dantrolene. Mayo Clin Proc 1995; 70: 241–6. Идроциламид Multi-ingredient: Turk.: Dorsilon. 5. Litman RS, Rosenberg H. Malignant hyperthermia: update on susceptibility testing. JAMA 2005; 293: 2918–24. C11H13NO2 = 191.2. 6. Rosenberg H, Gronert GA. Intractable cardiac arrest in children CAS — 6961-46-2. given succinylcholine. Anesthesiology 1992; 77: 1054. Metaxalone (BAN, USAN, rINN) Neuroleptic malignant syndrome. Dantrolene has been AHR-438; Metaxalona; Métaxalone; Metaxalonum. 5-(3,5-Xyly- used, usually alone or with bromocriptine, in the treatment of loxymethyl)oxazolidin-2-one. neuroleptic malignant syndrome (p.972), although some workers H Метаксалон 1 N have not found it to be of use, and evidence from controlled tri- C12H15NO3 = 221.3. als is lacking.2 Doses reported for dantrolene have varied great- OH CAS — 1665-48-1. 3,4 ly. For those patients unable to swallow and when rapid control O of symptoms is required, doses of 1 mg/kg or more have been given initially by intravenous injection. Up to 600 mg has been H3C given daily by mouth in divided doses. Adverse Effects 1. Rosebush PI, et al. The treatment of neuroleptic malignant syn- When given by mouth idrocilamide was reported to produce ab- dominal pain, nausea, and drowsiness. -

Review of Existing Classification Efforts
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.1.1 Review of existing classification efforts Due date of deliverable: (15.01.2008) Actual submission date: (07.02.2008) Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UGent Revision 1.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public X PP Restricted to other programme participants (including the Commission Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) Task 4.1 : Review of existing classification efforts Authors: Kristof Pil, Elke Raes, Thomas Van den Neste, An-Sofie Goessaert, Jolien Veramme, Alain Verstraete (Ghent University, Belgium) Partners: - F. Javier Alvarez (work package leader), M. Trinidad Gómez-Talegón, Inmaculada Fierro (University of Valladolid, Spain) - Monica Colas, Juan Carlos Gonzalez-Luque (DGT, Spain) - Han de Gier, Sylvia Hummel, Sholeh Mobaser (University of Groningen, the Netherlands) - Martina Albrecht, Michael Heiβing (Bundesanstalt für Straßenwesen, Germany) - Michel Mallaret, Charles Mercier-Guyon (University of Grenoble, Centre Regional de Pharmacovigilance, France) - Vassilis Papakostopoulos, Villy Portouli, Andriani Mousadakou (Centre for Research and Technology Hellas, Greece) DRUID 6th Framework Programme Deliverable D.4.1.1. Revision 1.0 Review of Existing Classification Efforts Page 2 of 127 Introduction DRUID work package 4 focusses on the classification and labeling of medicinal drugs according to their influence on driving performance.