Lorzone (Chlorzoxazone) Dose, Indications, Adverse Effects, Interactions... from PDR.Net 19/7/17 0:18
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHLORZOXAZONE- Chlorzoxazone Tablet DIRECT RX ------CHLORZOXAZONE
CHLORZOXAZONE- chlorzoxazone tablet DIRECT RX ---------- CHLORZOXAZONE DESCRIPTION SECTION Chlorzoxazone USP is a centrally acting skeletal muscle relaxant, available as tablets of 500 mg for oral administration. Its chemical name is 5-Chloro-2-benzoxazolinone, and its structural formula is: C7H4CINO2 MW 169.57 Chlorzoxazone USP is a white or practically white, practically odorless, crystalline powder. Chlorzoxazone is slightly soluble in water; sparingly soluble in alcohol, in isopropyl alcohol, and in methanol; soluble in solutions of alkali hydroxides and ammonia. Chlorzoxazone tablets contain the inactive ingredients Docusate Sodium, Lactose (hydrous), Magnesium Stearate, Microcrystalline Cellulose, Pregelatinized Starch, Sodium Benzoate, and Sodium Starch Glycolate. CLINICAL PHARMACOLOGY SECTION Chlorzoxazone is a centrally-acting agent for painful musculoskeletal conditions. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the level of the spinal cord and subcortical areas of the brain where it inhibits multisynaptic reflex arcs involved in producing and maintaining skeletal muscle spasm of varied etiology. The clinical result is a reduction of the skeletal muscle spasm with relief of pain and increased mobility of the involved muscles. Blood levels of chlorzoxazone can be detected in people during the first 30 minutes and peak levels may be reached, in the majority of the subjects, in about 1 to 2 hours after oral administration of chlorzoxazone. Chlorzoxazone is rapidly metabolized and is excreted in the urine, primarily in a conjugated form as the glucuronide. Less than one percent of a dose of chlorzoxazone is excreted unchanged in the urine in 24 hours. INDICATIONS & USAGE SECTION Chlorzoxazone is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. -

LORZONE- Chlorzoxazone Tablet Vertical Pharmaceuticals , LLC ---For Painful Musculoskeletal Conditions PRESCRIBING INFOR
LORZONE- chlorzoxazone tablet Vertical Pharmaceuticals , LLC ---------- For Painful Musculoskeletal Conditions PRESCRIBING INFORMATION DESCRIPTION Each 375 mg Lorzone® tablet contains: chlorzoxazone USP 375 mg. Each 750 mg Lorzone® tablet contains: chlorzoxazone USP 750 mg. Chemical Name: 5-Chloro-2-benzoxazolinone. Structural Formula: Molecular Formula: C7H4CINO2 Molecular Weight: 169.56 Chlorzoxazone USP is a white or practically white, practically odorless, crystalline powder. Chlorzoxazone is slightly soluble in water; sparingly soluble in alcohol, in isopropyl alcohol, and in methanol; soluble in solutions of alkali hydroxides and ammonia. Inactive ingredients: anhydrous lactose, croscarmellose sodium, docusate sodium, magnesium stearate, microcrystalline cellulose, pregelatinized corn starch and sodium benzoate. CLINICAL PHARMACOLOGY Chlorzoxazone is a centrally-acting agent for painful musculoskeletal conditions. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the level of the spinal cord and subcortical areas of the brain where it inhibits multisynaptic reflex arcs involved in producing and maintaining skeletal muscle spasm of varied etiology. The clinical result is a reduction of the skeletal muscle spasm with relief of pain and increased mobility of the involved muscles. Blood levels of chlorzoxazone can be detected in people during the first 30 minutes and peak levels may be reached, in the majority of the subjects, in about 1 to 2 hours after oral administration of chlorzoxazone. Chlorzoxazone is rapidly metabolized and is excreted in the urine, primarily in a conjugated form as the glucuronide. Less than one percent of a dose of chlorzoxazone is excreted unchanged in the urine in 24 hours. INDICATIONS AND USAGE Lorzone® is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. -

Case Report Carbamazepine Toxicity Following Oxybutynin And
Spinal Cord (2005) 43, 252–255 & 2005 International Spinal Cord Society All rights reserved 1362-4393/05 $30.00 www.nature.com/sc Case Report Carbamazepine toxicity following Oxybutynin and Dantrolene administration: a case report T Vander*,1, H Odi1, V Bluvstein1, J Ronen1,2 and A Catz1,2 1Department of Spinal Rehabilitation, Loewenstein Rehabilitation Hospital, Raanana, Israel; 2Tel-Aviv University, Tel-Aviv, Israel Objective: To report a case of Carbamazepine toxicity following the administration of Oxybutynin and Dantrolene. Study design: A case report. Setting: The Spinal Rehabilitation Department, Loewenstein Hospital, Raanana, Israel. Methods: A patient with C6D tetraplegia who sustained intoxication because of drug interaction is presented. She had been treated by Carbamazepine 1000 mg/day for neuropathic pain for 2 years without clinical or laboratory signs of toxicity. After administration of Oxybutynin concomitantly with an increase in the dose of Dantrolene, she presented the clinical symptoms and laboratory finding of Carbamazepine intoxication. Trying to adjust the treatment to the patient’s requirements, Carbamazepine together with Oxybutynin and Dantrolene was readministrated in lower doses. Results: The combination of these drugs, even small doses, caused toxicity. Adding Dantrolene and Oxybutynin elevated the blood level of Carbamazepine, possibly by inhibition of cytochrome P450. Conclusion: A possible pharmacokinetic interaction between Dantrolene and Oxybutynin should be borne in mind when considering Carbamazepine medication for a patient with a spinal cord lesion. Spinal Cord (2005) 43, 252–255. doi:10.1038/sj.sc.3101689; Published online 1 February 2005 Keywords: Carbamazepine; Oxybutynin; Dantrolene; drug interaction; cytochrome P450 Introduction Both Oxybutynin chloride and Dantrolene sodium are be required when patients have concomitant post- widely used in patients with spinal cord lesion (SCL). -

Skeletal Muscle Relaxants Review 12/17/2008
Skeletal Muscle Relaxants Review 12/17/2008 Copyright © 2007 - 2008 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 5181 Natorp Blvd., Suite 205 Mason, Ohio 45040 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to -

Drug Class Review on Skeletal Muscle Relaxants
Drug Class Review on Skeletal Muscle Relaxants Final Report Update 2 May 2005 Original Report Date: April 2003 Update 1 Report Date: January 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Roger Chou, MD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2005 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS Introduction........................................................................................................................4 Scope and -

San Juan County Adult Drug Court Participant Handbook
Participant Manual SEVENTH DISTRICT ADULT DRUG COURT MONTICELLO, UTAH Updated January 2018 Subject to Change 1 2 Welcome to the San Juan County Adult Drug Court This Handbook is designed to introduce you to the San Juan County Drug Court program, answer your questions and provide overall information about the Drug Court Program. As a participant, you will be expected to follow the instructions given in Drug Court by the Judge and comply with the treatment plan developed for you by the treatment team. If you are reading this Handbook it means that we are confident that Drug Court will help you to learn how to make successful choices free of the influence of drugs or alcohol. 3 Table of Contents Welcome to the San Juan County Adult Drug Court ...................................................................... 3 Overview ....................................................................................................................................... 6 Drug Court Team ........................................................................................................................... 7 Judge’s Role ........................................................................................................................... 7 San Juan County Attorney’s Role (Prosecutor) ....................................................................... 8 Defense Attorney Role (Your Attorney) ................................................................................... 8 Probation Officer’s Role ......................................................................................................... -

Anaesthetic Implications of Calcium Channel Blockers
436 Anaesthetic implications of calcium channel Leonard C. Jenkins aA MD CM FRCPC blockers Peter J. Scoates a sc MD FRCPC CONTENTS The object of this review is to emphasize the anaesthetic implications of calcium channel block- Physiology - calcium/calcium channel blockers Uses of calcium channel blockers ers for the practising anaesthetist. These drugs have Traditional played an expanding role in therapeutics since their Angina pectoris introduction and thus anaesthetists can expect to see Arrhythmias increasing numbers of patients presenting for anaes- Hypertension thesia who are being treated with calcium channel Newer and investigational Cardiac blockers. Other reviews have emphasized the basic - Hypertrophic cardiomyopathy pharmacology of calcium channel blockers. 1-7 - Cold cardioplegia - Pulmonary hypertension Physiology - calcium/calcium channel blockers Actions on platelets Calcium plays an important role in many physio- Asthma Obstetrics logical processes, such as blood coagulation, en- - Premature labor zyme systems, muscle contraction, bone metabo- - Pre-eclampsia lism, synaptic transmission, and cell membrane Achalasia and oesophageal spasm excitability. Especially important is the role of Increased intraocular pressure therapy calcium in myocardial contractility and conduction Protective effect on kidney after radiocontrast Cerebral vasospasm as well as in vascular smooth muscle reactivity. 7 Induced hypotensive anaesthesia Thus, it can be anticipated that any drug interfering Drag interactions with calcium channel blockers with the action of calcium could have widespread With anaesthetic agents effects. Inhalation agents In order to understand the importance of calcium - Effect on haemodynamics - Effect on MAC in cellular excitation, it is necessary to review some Neuromuscular blockers membrane physiology. Cell membranes are pri- Effects on epinephrine-induced arrhythmias marily phospholipids arranged in a bilayer. -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Original Research Article Comparison of Different Brands of Centrally Acting Skeletal Muscle Relaxants: a Cost Analysis Study
Print ISSN: 2319-2003 | Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20192213 Original Research Article Comparison of different brands of centrally acting skeletal muscle relaxants: a cost analysis study Kiran B., Kala P.*, Chitra N. S., Jamuna Rani R.* Department of Pharmacology, ABSTRACT SRM Medical College and Research Centre, Background: Skeletal muscle relaxants are structurally distinct drugs prescribed Kattankulathur, for reducing muscle spasms, pain, and hyperreflexia. Centrally acting skeletal Kancheepuram, Tamil Nadu, muscle relaxants are manufactured by various pharmaceutical companies with India variable price. The present study, aimed to analyze the cost variation of various brands of centrally acting skeletal muscle relaxants, so as to help the physician to Received: 15 April 2019 choose the cost effective treatment. Accepted: 13 May 2019 Methods: Current index of medical stores (CIMS) April 2018 and online literature were used as information guide to review the prices of drugs used in the *Correspondence to: treatment of musculo skeletal pain and spastic neurological disorders. Dr. Kala P., Results: Among anti spasmodic group, thiocolchicoside 4 mg shows maximum Email: kalaramesh75@ price variation of 337.5%, whereas carisoprodol 350 mg shows the least variation gmail.com of 0.1%. It is evident from antispastic group that baclofen 10 mg shows maximum price variation of 93.91% and 5 mg of Baclofen shows the least variation of Copyright: © the author(s), 11.22%. It is observed that, among anti spastic group, a percentage prize variation publisher and licensee Medip of 93.91 for 10 mg and 11.22 for 5 mg baclofen. -

Eagling Differential Selectivity of CYP Inhibitors Against Probe Substrates
Br J Clin Pharmacol 1998; 45: 107–114 Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes Victoria A. Eagling, John F. Tjia & David J. Back Department of Pharmacology & Therapeutics, University of Liverpool, P.O. Box 147, Liverpool L69 3GE, UK Aims Chemical inhibitors of cytochrome P450 (CYP) are a useful tool in defining the role of individual CYPs involved in drug metabolism. The aim of the present study was to evaluate the selectivity and rank the order of potency of a range of isoform-selective CYP inhibitors and to compare directly the eVects of these inhibitors in human and rat hepatic microsomes. Methods Four chemical inhibitors of human cytochrome P450 isoforms, furafylline (CYP1A2), sulphaphenazole (CYP2C9), diethyldithiocarbamate (CYP2E1), and ketoconazole (CYP3A4) were screened for their inhibitory specificity towards CYP- mediated reactions in both human and rat liver microsomal preparations. Phenacetin O-deethylation, tolbutamide 4-hydroxylation, chlorzoxazone 6-hydroxylation and testosterone 6b-hydroxylation were monitored for enzyme activity. Results Furafylline was a potent, selective inhibitor of phenacetin O-deethylation = (CYP1A2-mediated) in human liver microsomes (IC50 0.48 mm), but inhibited both phenacetin O-deethylation and tolbutamide 4-hydroxylation (CYP2C9- mediated) at equimolar concentrations in rat liver microsomes (IC50=20.8 and 24.0 mm respectively). Sulphaphenazole demonstrated selective inhibition of tolbutamide hydroxylation in human liver microsomes but failed to inhibit this reaction in rat liver microsomes. DDC demonstrated a low level of selectivity as an inhibitory probe for chlorzoxazone 6-hydroxylation (CYP2E1-mediated). DDC also inhibited testosterone 6b-hydroxylation (CYP3A-mediated) in man and rat, and tolbutamide 4-hydroxylase activity in rat. -

Cyclobenzaprine and Back Pain: a Meta-Analysis
ORIGINAL INVESTIGATION Cyclobenzaprine and Back Pain A Meta-analysis Robert Browning, MD; Jeffrey L. Jackson, MD, MPH; Patrick G. O’Malley, MD, MPH Background: Back pain is a common problem for which 14 as were those treated with placebo. Slightly fewer than cyclobenzaprine hydrochloride is frequently pre- 3 individuals (2.7; 95% confidence interval, 2.0-4.2) scribed. needed treatment for 1 to improve. The magnitude of this improvement was modest, with an effect size of 0.38 to Objective: To perform a systematic review of cyclo- 0.58 in all 5 outcomes (local pain, muscle spasm, ten- benzaprine’s effectiveness in the treatment of back pain. derness to palpation, range of motion, and activities of daily living). Treatment efficacy for these 5 outcomes was Methods: We searched MEDLINE, PsycLIT, CINAHL, greatest early, in the first few days of treatment, declin- EMBASE, AIDSLINE, HEALTHSTAR, CANCERLIT, the ing after the first week. Patients receiving cyclobenzap- Cochrane Library, Micromedex, Federal Research in rine also experienced more adverse effects, the most com- Progress, and the references of reviewed articles, and con- mon being drowsiness. tacted Merck, Sharpe and Dohme for English-language, randomized, placebo-controlled trials of cyclobenzap- Conclusions: Cyclobenzaprine is more effective than rine in adults with back pain. Outcomes included global placebo in the management of back pain; the effect is improvement and 5 specific domains of back pain (local modest and comes at the price of greater adverse effects. pain, muscle spasm, range of motion, tenderness to pal- The effect is greatest in the first 4 days of treatment, sug- pation, and activities of daily living). -
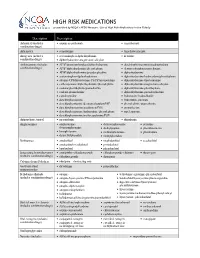
HIGH RISK MEDICATIONS As Specified by NCQA’S HEDIS Measure: Use of High Risk Medications in the Elderly
HIGH RISK MEDICATIONS as specified by NCQA’s HEDIS Measure: Use of High Risk Medications in the Elderly Description Prescription Antianxiety (includes • aspirin-meprobamate • meprobamate combination drugs) Antiemetics • scopolamine • trimethobenzamide Analgesics (includes • acetaminophen-diphenhydramine • ketorolac combination drugs) • diphenhydramine-magnesium salicylate Antihistamines (includes • APAP/dextromethorphan/diphenhydramine • dexchlorpheniramine-pseudoephedrine combination drugs) • APAP/diphenhydramine/phenylephrine • dextromethorphan-promethazine • APAP/diphenhydramine/pseudoephedrine • diphenhydramine • acetaminophen-diphenhydramine • diphenhydramine/hydrocodone/phenylephrine • atropine/CPM/hyoscyamine/PE/PPA/scopolamine • diphenhydramine-tripelennamine • carbetapentane/diphenhydramine/phenylephrine • diphenhydramine-magnesium salicylate • codeine/phenylephrine/promethazine • diphenhydramine-phenylephrine • codeine-promethazine • diphenhydramine-pseudoephedrine • cyproheptadine • hydroxyzine hydrochloride • dexchlorpheniramine • hydroxyzine pamoate • dexchlorpheniramine/dextromethorphan/PSE • phenylephrine-promethazine • dexchlorpheniramine/guaifenesin/PSE • promethazine • dexchlorpheniramine/hydrocodone/phenylephrine • tripelennamine • dexchlorpheniramine/methscopolamine/PSE Antipsychotic, typical • mesoridazine • thioridazine Amphetamines • amphetamine- • dextroamphetamine • pemoline dextroamphetamine • diethylpropion • phendimetrazine • benzphetamine • methamphetamine • phentermine • dexmethylphenidate • methylphenidate