Snake Antivenoms Adverse Reactions and Producer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
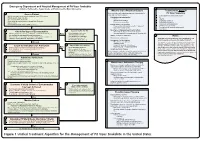
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Long-Term Effects of Snake Envenoming
toxins Review Long-Term Effects of Snake Envenoming Subodha Waiddyanatha 1,2, Anjana Silva 1,2 , Sisira Siribaddana 1 and Geoffrey K. Isbister 2,3,* 1 Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka, Saliyapura 50008, Sri Lanka; [email protected] (S.W.); [email protected] (A.S.); [email protected] (S.S.) 2 South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, University of Peradeniya, Peradeniya 20400, Sri Lanka 3 Clinical Toxicology Research Group, University of Newcastle, Callaghan, NSW 2308, Australia * Correspondence: [email protected] or [email protected]; Tel.: +612-4921-1211 Received: 14 March 2019; Accepted: 29 March 2019; Published: 31 March 2019 Abstract: Long-term effects of envenoming compromise the quality of life of the survivors of snakebite. We searched MEDLINE (from 1946) and EMBASE (from 1947) until October 2018 for clinical literature on the long-term effects of snake envenoming using different combinations of search terms. We classified conditions that last or appear more than six weeks following envenoming as long term or delayed effects of envenoming. Of 257 records identified, 51 articles describe the long-term effects of snake envenoming and were reviewed. Disability due to amputations, deformities, contracture formation, and chronic ulceration, rarely with malignant change, have resulted from local necrosis due to bites mainly from African and Asian cobras, and Central and South American Pit-vipers. Progression of acute kidney injury into chronic renal failure in Russell’s viper bites has been reported in several studies from India and Sri Lanka. Neuromuscular toxicity does not appear to result in long-term effects. -

Snakebite: the World's Biggest Hidden Health Crisis
Snakebite: The world's biggest hidden health crisis Snakebite is a potentially life-threatening neglected tropical disease (NTD) that is responsible for immense suffering among some 5.8 billion people who are at risk of encountering a venomous snake. The human cost of snakebite Snakebite Treatment Timeline Each year, approximately 5.4 million people are bitten by a snake, of whom 2.7 million are injected with venom. The first snake antivenom This leads to 400,000 people being permanently dis- produced, against the Indian Cobra. abled and between 83,000-138,000 deaths annually, Immunotherapy with animal- mostly in sub-Saharan Africa and South Asia. 1895 derived antivenom has continued to be the main treatment for snakebite evenoming for 120 years Snakebite: both a consequence and a cause of tropical poverty The Fav-Afrique antivenom, 2014 produced by Sanofi Pasteur (France) Survivors of untreated envenoming may be left with permanently discontinued amputation, blindness, mental health issues, and other forms of disability that severely affect their productivity. World Health Organization Most victims are agricultural workers and children in 2018 (WHO) lists snakebite envenoming the poorest parts of Africa and Asia. The economic as a neglected tropical disease cost of treating snakebite envenoming is unimaginable in most communities and puts families and communi- ties at risk of economic peril just to pay for treatment. WHO launches a strategy to prevent and control snakebite envenoming, including a program targeting affected communities and their health systems Global antivenom crisis 2019 The world produces less than half of the antivenom it The Scientific Research Partnership needs, and this only covers 57% of the world’s species for Neglected Tropical Snakbites of venomous snake. -

Bitis Arietans) Venom, and Their Neutralization by Antivenom
Toxins 2014, 6, 1586-1597; doi:10.3390/toxins6051586 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Article In Vitro Toxic Effects of Puff Adder (Bitis arietans) Venom, and Their Neutralization by Antivenom Steven Fernandez 1, Wayne Hodgson 1, Janeyuth Chaisakul 2, Rachelle Kornhauser 1, Nicki Konstantakopoulos 1, Alexander Ian Smith 3 and Sanjaya Kuruppu 3,* 1 Department of Pharmacology, Monash University, Building 13E, Wellington Road, Clayton, Vic 3800, Australia; E-Mails: [email protected] (S.F.); [email protected] (W.H.); [email protected] (R.K.); [email protected] (N.K.) 2 Department of Pharmacology, Phramongkutklao College of Medicine, Bangkok 10400, Thailand; E-Mail: [email protected] 3 Department of Biochemistry & Molecular Biology, Monash University, Building 77, Wellington Road, Clayton, Vic 3800, Australia; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +61-3-9902-9372; Fax: + 61-3-9902-9500. Received: 24 November 2013; in revised form: 6 April 2014 / Accepted: 4 May 2014 / Published: 19 May 2014 Abstract: This study investigated the in vitro toxic effects of Bitis arietans venom and the ability of antivenom produced by the South African Institute of Medical Research (SAIMR) to neutralize these effects. The venom (50 µg/mL) reduced nerve-mediated twitches of the chick biventer muscle to 19% ± 2% of initial magnitude (n = 4) within 2 h. This inhibitory effect of the venom was significantly attenuated by prior incubation of tissues with SAIMR antivenom (0.864 µg/µL; 67% ± 4%; P < 0.05; n = 3–5, unpaired t-test). -

Medicines/Pharmaceuticals of Animal Origin V3.0 November 2020
Medicines/pharmaceuticals of animal origin V3.0 November 2020 Medicines/pharmaceuticals of animal origin - This guideline provides information for all clinical staff within Hospital and Health Services (HHS) on best practice for avoidance of issues related to animal products. Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Published by the State of Queensland (Queensland Health), November 2020 This document is licensed under a Creative Commons Attribution 3.0 Australia licence. To view a copy of this licence, visit creativecommons.org/licenses/by/3.0/au © State of Queensland (Queensland Health) 2020 You are free to copy, communicate and adapt the work, as long as you attribute the State of Queensland (Queensland Health). For more information contact: Medication Services Queensland, Queensland Health, GPO Box 48, Brisbane QLD 4001, email [email protected] An electronic version of this document is available at https://www.health.qld.gov.au/__data/assets/pdf_file/0024/147507/qh-gdl-954.pdf Disclaimer: The content presented in this publication is distributed by the Queensland Government as an information source only. The State of Queensland makes no statements, representations or warranties about the accuracy, completeness or reliability of any information contained in this publication. The State of Queensland disclaims all responsibility and all liability (including without limitation for liability in negligence) for all expenses, losses, damages and costs you might incur as a result of the information being inaccurate -

Epidemiology and Clinical Outcomes of Snakebite in the Elderly: a Toxic Database Study
Clinical Toxicology ISSN: 1556-3650 (Print) 1556-9519 (Online) Journal homepage: http://www.tandfonline.com/loi/ictx20 Epidemiology and clinical outcomes of snakebite in the elderly: a ToxIC database study Meghan B. Spyres, Anne-Michelle Ruha, Kurt Kleinschmidt, Rais Vohra, Eric Smith & Angela Padilla-Jones To cite this article: Meghan B. Spyres, Anne-Michelle Ruha, Kurt Kleinschmidt, Rais Vohra, Eric Smith & Angela Padilla-Jones (2018) Epidemiology and clinical outcomes of snakebite in the elderly: a ToxIC database study, Clinical Toxicology, 56:2, 108-112, DOI: 10.1080/15563650.2017.1342829 To link to this article: https://doi.org/10.1080/15563650.2017.1342829 Published online: 13 Jul 2017. Submit your article to this journal Article views: 120 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ictx20 CLINICAL TOXICOLOGY, 2018 VOL. 56, NO. 2, 108–112 https://doi.org/10.1080/15563650.2017.1342829 CLINICAL RESEARCH Epidemiology and clinical outcomes of snakebite in the elderly: a ToxIC Ã database study Meghan B. Spyresa, Anne-Michelle Ruhab, Kurt Kleinschmidtc, Rais Vohrad, Eric Smithc and Angela Padilla-Jonesb aDepartment of Emergency Medicine, Division of Medical Toxicology, University of Southern California, Los Angeles, CA, USA; bDepartment of Medical Toxicology, Banner-University Medical Center Phoenix, Phoenix, AZ, USA; cDepartment of Emergency Medicine, Univesity of Texas Southwestern Medical Center, Dallas, TX, USA; dDepartment of Emergency Medicine, UCSF Fresno Medical Center, Fresno, CA, USA ABSTRACT ARTICLE HISTORY Introduction: Epidemiologic studies of snakebites in the United States report typical victims to be Received 23 February 2017 young men. -

Polyvalent Horse F(Ab`)2 Snake Antivenom: Development of Process to Produce Polyvalent Horse F(Ab`)2 Antibodies Anti-African Snake Venom
African Journal of Biotechnology Vol. 9 (16), pp. 2446-2455, 19 April, 2010 Available online at http://www.academicjournals.org/AJB ISSN 1684–5315 © 2009 Academic Journals Full Length Research Paper Polyvalent horse F(Ab`)2 snake antivenom: Development of process to produce polyvalent horse F(Ab`)2 antibodies anti-african snake venom R. G. Guidlolin1, R. M. Marcelino1, H. H. Gondo1, J. F. Morais1, R. A. Ferreira2, C. L. Silva2, T. L. Kipnis2, J. A. Silva2, J. Fafetine3 and W. D. da Silva2* 1Divisao Bioindustrial, Instituto Butantan, Sao Paulo, SP, Brazil. 2Laboratório de Biologia do Reconhecer, Centro de Biociencias e Biotecnologia, Universidade Estadual do Norte Fluminense – Darcy Ribeiro, Campos dos Goytacazes, RJ, Brazil. 3Universidade Eduardo Mondlane, Maputo, Mozambique. Accepted 23 March, 2010 A method to obtain polyvalent anti-Bitis and polyvalent-anti-Naja antibodies was developed by immunizing horses with B. arietans, B. nasicornis, B. rhinoceros, N. melanoleuca and N. mossambica crude venoms. Antibody production was followed by the ELISA method during the immunization procedure. Once the desired anti-venom antibody titers were attained, horses were bled and the immunoglobulins were separated from the sera by (NH4)2SO4 precipitation, cleaved with pepsin and filtered through a 30 kDa ultrafiltration membrane. F(ab´)2 fragments were further purified by Q-Fast Flow chromatography, concentrated by molecular ultrafiltration and sterilized by filtration through 0.22 m membranes. The resulting F(ab´)2 preparations were rich in intact L and in pieces of H IgG(T) chains, as demonstrated by electrophoresis and Western blot and exhibited high antibody titers, as assayed by the ELISA method. -

Snakebite Management in Nepal
NATIONAL GUIDELINES FOR SNAKEBITE MANAGEMENT IN NEPAL 2019 Government of Nepal Ministry of Health and Population Department of Health Services Epidemiology and Disease Control Division Teku, Kathmandu NATIONAL GUIDELINES FOR SNAKEBITE MANAGEMENT IN NEPAL Government of Nepal Ministry of Health and Population Department of Health Services Epidemiology and Disease Control Division Teku, Kathmandu ii NATIONAL GUIDELINES FOR SNAKEBITE MANAGEMENT IN NEPAL CONTENTS 1 INTRODUCTION 1 2 SNAKES OF MEDICAL IMPORTANCE IN NEPAL 3 2.1 Elapidae Family 4 2.2 Viperidae Family 9 3 CLINICAL MANIFESTATION OF COMMON VENOMOUS SNAKES OF NEPAL 17 3.1 Local manifestations 18 3.2 Systemic manifestations 19 3.3 Clinical syndrome of snakebite envenoming in Nepal 27 3.4 Long term complications (sequelae) of snakebite envenoming 28 4 DIAGNOSIS OF SNAKEBITE ENVENOMING 29 5 MANAGEMENT OF SNAKEBITE ENVENOMING 31 5.1 First aid treatment and transport to the hospital 31 5.2 Rapid clinical assessment and resuscitation 36 5.3 Antivenom treatment 37 5.4 Supportive/ancillary treatment 42 5.5 Treatment of the bitten part 43 6 REFERRAL OF SNAKEBITE PATIENTS 45 7 ROLE OF DIFFERENT LEVEL OF HEALTH SERVICES FOR SNAKEBITE MANAGEMENT 47 8 MANAGEMENT OF SNAKEBITE ENVENOMING WHEN NO ANTIVENOM IS AVAILABLE 49 9 PREVENTION OF SNAKEBITE 51 iii NATIONAL GUIDELINES FOR SNAKEBITE MANAGEMENT IN NEPAL ANNEXES Annexe 1: 20 minutes whole blood clotting test (20wbct) 58 Annexe 2: Treatment of antivenom reactions 60 Annexe 3: Airway protection and management 64 Annexe 4: Treatment of hypotension -

Draft: Primary Health Care Services
11/27/2019 DRAFT: PRIMARY HEALTH CARE SERVICES COUNCIL FOR MEDICAL SCHEMES Disclaimer The proposed set of services and interventions are a first step towards defining services that should be available in a primary health care setting. It should be noted that at this stage, these proposals comprise inputs by different stakeholders and do not represent the final views of the PMB Review Advisory Committee. Page 1 of 109 Acknowledgements This draft document is the culmination of a task that has involved many stakeholders. It benefitted greatly from ideas, inputs and review from a broad range of participation from providers, academia, funders, professional bodies and consumers. A special thanks to the PMB Review Advisory Committee for providing guidance on the review of the PMBs. A special tribute is paid to the clinical experts who contributed their time and experience to review the proposed package. Without their contributions, the Council for Medical Schemes would not have achieved this important milestone. The project was co-ordinated for the Council for Medical Schemes, by the Clinical Unit. Page 2 of 109 Abbreviations AIDS Acquired Immune Deficiency Syndrome CMS Council for Medical Schemes DALYs Disability Adjusted Life Years DCSTs District Specialist Teams DTPs Diagnosis Treatment Pairs EPI Expanded Program on Immunisation GDP Gross Domestic Product HIV Human Immunodeficiency Virus MCDA Multiple Criteria Decision Analysis MDGs Millennium Developmental Goals MDT Multi-Disciplinary Teams MMSE Mini-Mental State Examination MOCA Montreal Cognitive Assessment NDP National Developmental Plan NDoH National Department of Health NHI National Health Insurance NPC National Planning Commission PHC Primary Health Care PMBs Prescribed Minimum Benefits SDGs Sustainable Development Goals StatsSA Statistics South Africa TB Tuberculosis UHC Universal Health Coverage Page 3 of 109 Table of Contents Disclaimer ............................................................................................................................................................. -

The Selection and Use of Essential Medicines
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the World Health Organization WHO Technical Report Series 933 THE SELECTION AND USE OF ESSENTIAL MEDICINES Report of the WHO Expert Committee, 2005 (including the 14th Model List of Essential Medicines) World Health Organization Geneva 2006 i WHO Library Cataloguing-in-Publication Data WHO Expert Committee on the Selection and Use of Essential Medicines (14th : 2005: Geneva, Switzerland) The selection and use of essential medicines : report of the WHO Expert Committee, 2005 : (including the 14th model list of essential medicines). (WHO technical report series ; 933) 1.Essential drugs — standards 2.Formularies — standards 3.Drug information services — organization and administration 4.Drug utilization 5. Pharmaceutical preparations — classification 6.Guidelines I.Title II.Title: 14th model list of essential medicines III.Series. ISBN 92 4 120933 X (LC/NLM classification: QV 55) ISSN 0512-3054 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publica- tions — whether for sale or for noncommercial distribution — should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Comparison of Pasteur and Behringwerke Antivenoms In
BRITISH MEDICAL JOURNAL 1 MARCH 1980 607 Comparison of Pasteur and Behringwerke antivenoms in envenoming by the carpet viper (Echis carinatus) Br Med J: first published as 10.1136/bmj.280.6214.607 on 1 March 1980. Downloaded from D A WARRELL, M J WARRELL, W EDGAR, C R M PRENTICE, JERRELL MATHISON, JOYCE MATHISON Summary and conclusions In 1975 two of us began to achieve good results with the Pasteur Echis antivenom at Bambur General Hospital, which admits over Bites and envenoming by the carpet viper Echis carinatus 100 cases of Echis bite each year. This studyis a formal compari- are common medical emergencies in parts ofNigeria, but son of these two antivenoms. the most effective use of the various commercially produced antivenoms in treatment has not been estab- lished. Pasteur Paris Echis monospecific and Behring- werke West and North Africa Bitis-Echis-Naja poly- Patients and methods specific antivenoms were compared in two groups of seven patients with incoagulable blood after E carinatus Patients-Fourteen patients with snake bites admitted to Bambur General Hospital in May and June 1977 were found to have in- bites. In both groups spontaneous bleeding stopped coagulable blood, which in West Africa is diagnostic of envenoming a few hours and local swelling within two within subsided by E carinatus.2 In six cases the dead snake was brought in and found weeks after the initial antivenom injection. Pasteur anti- to be E carinatus ocellatus Stemmler; the average length was 60 cm venom (20-40 ml) restored blood coagulability within 12 (these specimens were deposited at the British Museum, Natural hours in all cases, but 60-180 ml of Behringwerke anti- History (accession numbers 1979 31-6)). -

Moringa Oleifera Extract Extenuates Echis Ocellatus Venom-Induced Toxicities, Histopathological Impairments and Inflammation Via Enhancement of Nrf2 Expression in Rats
Article Moringa oleifera Extract Extenuates Echis ocellatus Venom-Induced Toxicities, Histopathological Impairments and Inflammation via Enhancement of Nrf2 Expression in Rats Akindele O. Adeyi 1,*, Sodiq O. Adeyemi 1, Enoh-Obong P. Effiong 1, Babafemi S. Ajisebiola 2, Olubisi E. Adeyi 3 and Adewale S. James 3 1 Animal Physiology Unit, Department of Zoology, University of Ibadan, Ibadan P.M.B. 200284, Oyo State, Nigeria; [email protected] (S.O.A.); [email protected] (E.-O.P.E.) 2 Department of Zoology, Osun State University, Oshogbo P.M.B. 230212, Osun State, Nigeria; [email protected] 3 Department of Biochemistry, Federal University of Agriculture, Abeokuta P.M.B. 2240, Ogun State, Nigeria; [email protected] (O.E.A.); [email protected] (A.S.J.) * Correspondence: [email protected]; Tel.: +234-80-3069-2698 Abstract: Echis ocellatus snakebite causes more fatalities than all other African snake species combined. Moringa oleifera reportedly possesses an antivenom property. Therefore, we evaluated the effective- ness of M. oleifera ethanol extract (MOE) against E. ocellatus venom (EOV) toxicities. Thirty male rats were grouped as follows (n = 5): Group 1 (normal control received saline), groups 2 to 6 were administered intraperitoneally, 0.22 mg/kg (LD50) of EOV. Group 2 was left untreated while group 3 to 6 were treated post-envenoming with 0.2 mL of polyvalent antivenom, 200, 400, and 600 mg/kg of MOE respectively. MOE significantly (p < 0.05) normalized the altered haematological indices and Citation: Adeyi, A.O.; Adeyemi, blood electrolytes profiles. MOE attenuated venom-induced cellular dysfunctions, characterized by a S.O.; Effiong, E.-O.P.; Ajisebiola, B.S.; Adeyi, O.E.; James, A.S.