Long-Term Effects of Snake Envenoming
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Snake Bite Protocol
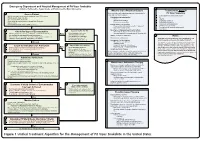
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Approach to the Poisoned Patient
PED-1407 Chocolate to Crystal Methamphetamine to the Cinnamon Challenge - Emergency Approach to the Intoxicated Child BLS 08 / ALS 75 / 1.5 CEU Target Audience: All Pediatric and adolescent ingestions are common reasons for 911 dispatches and emergency department visits. With greater availability of medications and drugs, healthcare professionals need to stay sharp on current trends in medical toxicology. This lecture examines mind altering substances, initial prehospital approach to toxicology and stabilization for transport, poison control center resources, and ultimate emergency department and intensive care management. Pediatric Toxicology Dr. James Burhop Pediatric Emergency Medicine Children’s Hospital of the Kings Daughters Objectives • Epidemiology • History of Poisoning • Review initial assessment of the child with a possible ingestion • General management principles for toxic exposures • Case Based (12 common pediatric cases) • Emerging drugs of abuse • Cathinones, Synthetics, Salvia, Maxy/MCAT, 25I, Kratom Epidemiology • 55 Poison Centers serving 295 million people • 2.3 million exposures in 2011 – 39% are children younger than 3 years – 52% in children younger than 6 years • 1-800-222-1222 2011 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System Introduction • 95% decline in the number of pediatric poisoning deaths since 1960 – child resistant packaging – heightened parental awareness – more sophisticated interventions – poison control centers Epidemiology • Unintentional (1-2 -

WHO Guidance on Management of Snakebites
GUIDELINES FOR THE MANAGEMENT OF SNAKEBITES 2nd Edition GUIDELINES FOR THE MANAGEMENT OF SNAKEBITES 2nd Edition 1. 2. 3. 4. ISBN 978-92-9022- © World Health Organization 2016 2nd Edition All rights reserved. Requests for publications, or for permission to reproduce or translate WHO publications, whether for sale or for noncommercial distribution, can be obtained from Publishing and Sales, World Health Organization, Regional Office for South-East Asia, Indraprastha Estate, Mahatma Gandhi Marg, New Delhi-110 002, India (fax: +91-11-23370197; e-mail: publications@ searo.who.int). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. -

Bibliography and Scientific Name Index to Amphibians
lb BIBLIOGRAPHY AND SCIENTIFIC NAME INDEX TO AMPHIBIANS AND REPTILES IN THE PUBLICATIONS OF THE BIOLOGICAL SOCIETY OF WASHINGTON BULLETIN 1-8, 1918-1988 AND PROCEEDINGS 1-100, 1882-1987 fi pp ERNEST A. LINER Houma, Louisiana SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 92 1992 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION The present alphabetical listing by author (s) covers all papers bearing on herpetology that have appeared in Volume 1-100, 1882-1987, of the Proceedings of the Biological Society of Washington and the four numbers of the Bulletin series concerning reference to amphibians and reptiles. From Volume 1 through 82 (in part) , the articles were issued as separates with only the volume number, page numbers and year printed on each. Articles in Volume 82 (in part) through 89 were issued with volume number, article number, page numbers and year. -

Approach and Management of Spider Bites for the Primary Care Physician
Osteopathic Family Physician (2011) 3, 149-153 Approach and management of spider bites for the primary care physician John Ashurst, DO,a Joe Sexton, MD,a Matt Cook, DOb From the Departments of aEmergency Medicine and bToxicology, Lehigh Valley Health Network, Allentown, PA. KEYWORDS: Summary The class Arachnida of the phylum Arthropoda comprises an estimated 100,000 species Spider bite; worldwide. However, only a handful of these species can cause clinical effects in humans because many Black widow; are unable to penetrate the skin, whereas others only inject prey-specific venom. The bite from a widow Brown recluse spider will produce local symptoms that include muscle spasm and systemic symptoms that resemble acute abdomen. The bite from a brown recluse locally will resemble a target lesion but will develop into an ulcerative, necrotic lesion over time. Spider bites can be prevented by several simple measures including home cleanliness and wearing the proper attire while working outdoors. Although most spider bites cause only local tissue swelling, early species identification coupled with species-specific man- agement may decrease the rate of morbidity associated with bites. © 2011 Elsevier Inc. All rights reserved. More than 100,000 species of spiders are found world- homes and yards in the southwestern United States, and wide. Persons seeking medical attention as a result of spider related species occur in the temperate climate zones across bites is estimated at 50,000 patients per year.1,2 Although the globe.2,5 The second, Lactrodectus, or the common almost all species of spiders possess some level of venom, widow spider, are found in both temperate and tropical 2 the majority are considered harmless to humans. -

An in Vivo Examination of the Differences Between Rapid
www.nature.com/scientificreports OPEN An in vivo examination of the diferences between rapid cardiovascular collapse and prolonged hypotension induced by snake venom Rahini Kakumanu1, Barbara K. Kemp-Harper1, Anjana Silva 1,2, Sanjaya Kuruppu3, Geofrey K. Isbister 1,4 & Wayne C. Hodgson1* We investigated the cardiovascular efects of venoms from seven medically important species of snakes: Australian Eastern Brown snake (Pseudonaja textilis), Sri Lankan Russell’s viper (Daboia russelii), Javanese Russell’s viper (D. siamensis), Gaboon viper (Bitis gabonica), Uracoan rattlesnake (Crotalus vegrandis), Carpet viper (Echis ocellatus) and Puf adder (Bitis arietans), and identifed two distinct patterns of efects: i.e. rapid cardiovascular collapse and prolonged hypotension. P. textilis (5 µg/kg, i.v.) and E. ocellatus (50 µg/kg, i.v.) venoms induced rapid (i.e. within 2 min) cardiovascular collapse in anaesthetised rats. P. textilis (20 mg/kg, i.m.) caused collapse within 10 min. D. russelii (100 µg/kg, i.v.) and D. siamensis (100 µg/kg, i.v.) venoms caused ‘prolonged hypotension’, characterised by a persistent decrease in blood pressure with recovery. D. russelii venom (50 mg/kg and 100 mg/kg, i.m.) also caused prolonged hypotension. A priming dose of P. textilis venom (2 µg/kg, i.v.) prevented collapse by E. ocellatus venom (50 µg/kg, i.v.), but had no signifcant efect on subsequent addition of D. russelii venom (1 mg/kg, i.v). Two priming doses (1 µg/kg, i.v.) of E. ocellatus venom prevented collapse by E. ocellatus venom (50 µg/kg, i.v.). B. gabonica, C. vegrandis and B. -

Snake Bite Prevention What to Do If You Are Bitten
SNAKE BITE PREVENTION It has been estimated that 7,000–8,000 people per year are bitten by venomous snakes in the United States, and for around half a dozen people, these bites are fatal. In 2015, poison centers managed over 3,000 cases of snake and other reptile bites during the summer months alone. Approximately 80% of these poison center calls originated from hospitals and other health care facilities. Venomous snakes found in the U.S. include rattlesnakes, copperheads, cottonmouths/water moccasins, and coral snakes. They can be especially dangerous to outdoor workers or people spending more time outside during the warmer months of the year. Most snakebites occur when people accidentally step on or come across a snake, frightening it and causing it to bite defensively. However, by taking extra precaution in snake-prone environments, many of these bites are preventable by using the following snakebite prevention tips: Avoid surprise encounters with snakes: Snakes tend to be active at night and in warm weather. They also tend to hide in places where they are not readily visible, so stay away from tall grass, piles of leaves, rocks, and brush, and avoid climbing on rocks or piles of wood where a snake may be hiding. When moving through tall grass or weeds, poke at the ground in front of you with a long stick to scare away snakes. Watch where you step and where you sit when outdoors. Shine a flashlight on your path when walking outside at night. Wear protective clothing: Wear loose, long pants and high, thick leather or rubber boots when spending time in places where snakes may be hiding. -
![Bites and Stings [Poisonous Animals and Plants]](https://docslib.b-cdn.net/cover/0546/bites-and-stings-poisonous-animals-and-plants-720546.webp)
Bites and Stings [Poisonous Animals and Plants]
Poisonous animals and plants Dr Tim Healing Dip.Clin.Micro, DMCC, CBIOL, FZS, FRSB Course Director, Course in Conflict and Catastrophe Medicine Worshipful Society of Apothecaries of London Faculty of Conflict and Catastrophe Medicine Animal and plant toxins • In most instances the numbers of people affected will be small • There are a few instances where larger numbers may be involved – mainly due to food-borne toxins Poisonous species (N.B. Venomous species use poisons for attack, poisonous animals and plants for passive defence) Venomous and poisonous animals – Reptiles (snakes) – Amphibians (dart frogs) – Arthropods (scorpions, spiders, wasps, bees, centipedes) – Aquatic animals (fish, jellyfish, octopi) Poisonous plants – Contact stinging (nettles, poison ivy, algae) – Poisonous by ingestion (fungi, berries of some plants) – Some algae Snakes Venomous Snakes • About 600 species of snake are venomous (ca. 25% of all snake species). Four main groups: – Elapidae (elapids). Mambas, Cobras, King cobras, Kraits, Taipans, Sea snakes, Brown snakes, Coral snakes. – Viperidae (viperids). True vipers and pit vipers (including Rattlesnakes, Copperheads and Cottonmouths) – Colubridae (colubrids). Mostly harmless, but includes the Boomslang – Atractaspididae (atractaspidids). Burrowing asps, Mole vipers, Stiletto snakes. Geographical distribution • Elapidae: – On land, worldwide in tropical and subtropical regions, except in Europe. – Sea snakes occur in the Indian Ocean and the Pacific • Viperidae: – The Americas, Africa and Eurasia. • Boomslangs -

Identifying Intraspecific Variation in Venom Yield of Chinese Cobra (Naja Atra) from Ten Populations in Mainland China
Asian Herpetological Research 2019, 10(1): 32–40 ORIGINAL ARTICLE DOI: 10.16373/j.cnki.ahr.180041 Identifying Intraspecific Variation in Venom Yield of Chinese Cobra (Naja atra) from Ten Populations in Mainland China Jianfang GAO1*, Yin YIN1, Yanfu QU2, Jin WANG2, Longhui LIN1, Hongliang LU1 and Xiang JI2 1 Hangzhou Key Laboratory for Animal Adaptation and Evolution, College of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou 310036, Zhejiang, China 2 Jiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University, Nanjing 210046, Jiangsu, China Abstract Detailed information on venom yield is helpful in preparing antivenoms and treating snakebites, but such information is lacking for many species of venomous snakes. The Chinese cobra (Naja atra) is a large sized, venomous snake commonly found in southeastern China, where it causes a heavy burden of snakebites. To examine the effects of various factors (morphology, sex, age, season, and geographical origin) on the venom yield in this snake, we collected venom samples of 446 individuals (426 adults and 20 neonates) from 10 populations of N. atra over an eight- year period. We used two variables, lyophilized venom mass (venom yield) and solid content of venom (% solids), to quantify the venom yield. We used linear regression analysis to check if venom yield was related to morphological factors, one-way ANOVA and one-way ANCOVA to detect the sexual, ontogenetic, and geographic variation in venom yield, and repeated-measures ANOVA to examine seasonal shifts in venom yield. Our results indicate that venom yield of N. atra is positively related to the morphological traits examined, with male snakes expelling more venom than females. -

Quantitative Characterization of the Hemorrhagic, Necrotic, Coagulation
Hindawi Journal of Toxicology Volume 2018, Article ID 6940798, 8 pages https://doi.org/10.1155/2018/6940798 Research Article Quantitative Characterization of the Hemorrhagic, Necrotic, Coagulation-Altering Properties and Edema-Forming Effects of Zebra Snake (Naja nigricincta nigricincta)Venom Erick Kandiwa,1 Borden Mushonga,1 Alaster Samkange ,1 and Ezequiel Fabiano2 1 School of Veterinary Medicine, Faculty of Agriculture and Natural Resources, Neudamm Campus, University of Namibia, P. Bag 13301, Pioneers Park, Windhoek, Namibia 2Department of Wildlife Management and Ecotourism, Katima Mulilo Campus, Faculty of Agriculture and Natural Resources, University of Namibia, P. Bag 1096, Ngweze, Katima Mulilo, Namibia Correspondence should be addressed to Alaster Samkange; [email protected] Received 30 May 2018; Revised 5 October 2018; Accepted 10 October 2018; Published 24 October 2018 Academic Editor: Anthony DeCaprio Copyright © 2018 Erick Kandiwa et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Tis study was designed to investigate the cytotoxicity and haemotoxicity of the Western barred (zebra) spitting cobra (Naja nigricincta nigricincta) venom to help explain atypical and inconsistent reports on syndromes by Namibian physicians treating victims of human ophidian accidents. Freeze-dried venom milked from adult zebra snakes was dissolved in phosphate bufered saline (PBS) for use in this study. Haemorrhagic and necrotic activity of venom were studied in New Zealand albino rabbits. Oedema-forming activity was investigated in 10-day-old Cobb500 broiler chicks. Procoagulant and thrombolytic activity was investigated in adult Kalahari red goat blood in vitro. -

Naja Atra) Bites: Determining Bacteriology, Antibiotic Susceptibility, and the Use of Antibiotics-A Cobra BITE Study
toxins Article Wound Infections from Taiwan Cobra (Naja atra) Bites: Determining Bacteriology, Antibiotic Susceptibility, and the Use of Antibiotics-A Cobra BITE Study Heng Yeh 1,2, Shi-Ying Gao 1 and Chih-Chuan Lin 1,2,* 1 Department of Emergency Medicine, Lin-Kou Medical Center, Chang Gung Memorial Hospital, Taoyuan 33305, Taiwan; [email protected] (H.Y.); [email protected] (S.-Y.G.) 2 School of Medicine, College of Medicine, Chang Gung University, Taoyuan 33302, Taiwan * Correspondence: [email protected] Abstract: Wound necrosis and secondary infection are common complications after Naja atra bites. Clinical tools to evaluate the infection risk after Taiwan cobra bites are lacking. In this Cobra BITE study, we investigated the prevalence of wound infection, bacteriology, and corresponding antibiotic usage in patients presenting with Taiwan cobra snakebites. Patients with wound infection lacking tissue necrosis were included in developing Cobra BITE score utilizing univariate and multiple logistic regression, as patients with wound necrosis require antibiotics for infection treatment. 8,295,497 emergency department visits occurred in the span of this study, with 195 of those patients being diagnosed as having cobra bites. Of these patients, 23 had wound necrosis, and 30 had wound infection, resulting in a wound infection rate of 27.2% (53/195). Enterococcus faecalis and Morganella morganii were the main bacteria identified in the culture report regardless of whether patients’ wounds had necrosis. As per our Cobra BITE score, the three factors predicting secondary wound infection after cobra bites are hospital admission, a white blood cell count (in 103/µL) × by neu-trophil-lymphocyte ratio value of ≥114.23, and the use of antivenin medication. -

Venom Evolution Widespread in Fishes: a Phylogenetic Road Map for the Bioprospecting of Piscine Venoms
Journal of Heredity 2006:97(3):206–217 ª The American Genetic Association. 2006. All rights reserved. doi:10.1093/jhered/esj034 For permissions, please email: [email protected]. Advance Access publication June 1, 2006 Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms WILLIAM LEO SMITH AND WARD C. WHEELER From the Department of Ecology, Evolution, and Environmental Biology, Columbia University, 1200 Amsterdam Avenue, New York, NY 10027 (Leo Smith); Division of Vertebrate Zoology (Ichthyology), American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Leo Smith); and Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Wheeler). Address correspondence to W. L. Smith at the address above, or e-mail: [email protected]. Abstract Knowledge of evolutionary relationships or phylogeny allows for effective predictions about the unstudied characteristics of species. These include the presence and biological activity of an organism’s venoms. To date, most venom bioprospecting has focused on snakes, resulting in six stroke and cancer treatment drugs that are nearing U.S. Food and Drug Administration review. Fishes, however, with thousands of venoms, represent an untapped resource of natural products. The first step in- volved in the efficient bioprospecting of these compounds is a phylogeny of venomous fishes. Here, we show the results of such an analysis and provide the first explicit suborder-level phylogeny for spiny-rayed fishes. The results, based on ;1.1 million aligned base pairs, suggest that, in contrast to previous estimates of 200 venomous fishes, .1,200 fishes in 12 clades should be presumed venomous.