Approach to the Poisoned Patient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

News and Notes 97
96 Injury Prevention 1998;4:96–97 ... while in Canada, helmet laws nated eVorts. It sets targets for the reduction Inj Prev: first published as 10.1136/ip.4.2.96 on 1 June 1998. Downloaded from knocked of deaths and injuries requiring hospitalisa- NEWS AND tion for traYc collisions, fires, drownings, and As summer approached, the debate over those in the workplace and home. Recognis- NOTES bicycle helmets in Canada was heating up. ing the lack of information on sport and rec- Countering a growing movement to get reational injuries the report notes that this helmet laws passed in every province is an area requires further attention. Further details: organization called the Ontario Coalition for Minister’s Injury Prevention Committee, c/o Better Cycling whose web site applauds the OYce for Injury Prevention, Environmental Revised injury control manual from failures of helmet law lobbyists and the Health Assessment and Safety Branch, BC Ministry of Health, 7-1 1515 Blanshard AAP watering down of Ontario’s law (only cyclists younger than 18 must wear helmets or face a Street, Victoria BC V8V 3C8, Canada (fax: The new injury control manual from the fine in that province). The group argues that +1 250 952 1342). American Academy of Pediatrics (AAP) is the government should instead mandate now available and by all accounts is well cycling education in schools, on the grounds Our Healthier Nation worth looking at. It contains new chapters on that more crashes and injuries can be violence, sports injuries, agricultural injuries, prevented “by getting children to appreciate A Contract for Health is how the UK govern- the need to cycle responsibly”. -

Drug Safety Oversight Board Members
April 29, 2021 Drug Safety Oversight Board (DSOB) Roster Chair • Douglas Throckmorton, M.D., Deputy Director for Regulatory Programs Center for Drug Evaluation and Research Executive Director • Terry Toigo, R.Ph, MBA Associate Director for Drug Safety Operations, Center for Drug Evaluation and Research Food and Drug Administration Center for Drug Evaluation and Research (CDER) Office of the Center Director (OCD) Primary Member: • Robert Temple, M.D., Deputy Director for Clinical Science Office of New Drugs (OND) Primary Member: • Mary Thanh Hai, M.D., Deputy Director, Office of New Drugs Alternate Members: • Peter Stein, M.D., Director, Office of New Drugs • Ellis Unger, M.D., Director, Office of Office of Cardiology, Hematology, Endocrinology, and Nephrology (OCHEN) Office of Medical Policy (OMP) Primary Member: • Jacqueline Corrigan-Curay, Director Alternate Member: • Leonard V. Sacks, Mgr. Supervisory Medical Officer Office of Generic Drugs (OGD) Primary Member: • Linda Forsyth, M.D., Division of Clinical Review Alternate Member: • Vacant Office of Surveillance and Epidemiology (OSE) Primary Members: • Mark I. Avigan, M.D., Associate Director for Critical Path Initiatives • Judy Zander, M.D., Director, Office of Pharmacoviligance and Epidemiology (OPE) Alternate Members: • Gerald DalPan, M.D., M.H.S., Director, OSE • S. Chris Jones, Deputy Director, Division of Pharmacovigilance (DPV) II • Judy Staffa, Ph.D., R.Ph., Associate Director for Public Health Initiatives -Page 1 of 4- April 29, 2021 • Cynthia LaCivita, R.Ph, Director, Division -

Symptom Management in the Last Days of Life This Is About Managing Symptoms in the Last Days of Life Where the Dying Process Has Set In
Symptom management in the last days of life This is about managing symptoms in the last days of life where the dying process has set in. It assumes that the therapeutic aims are therefore: To allow the patient to die comfortably To support the family/carers and to start to prepare them for bereavement To discontinue any burdensome or irrelevant clinical procedures Key Prescribing Questions in the last days of life 1. Ahead of time Page a. Pre-emptive prescribing 1 b. Differences in specific circumstances 1 2. Once the oral route is lost a. What can be stopped? – managing co-morbidities at the end of life 2 (insulin; anti-epileptics; steroids; cardiac medicines) b. How to prescribe a syringe pump 3 i. Opioid conversion 4 ii. Combining drugs in a syringe – what can and can’t be mixed? 5 iii. Dosing other drugs 6 c. What can and can’t be given subcutaneously 7 3. Problems a. Uncontrolled symptoms (pain; restlessness; secretions; breathlessness; nausea; thirst) 8 b. Obtaining medicines out of hours 11 c. References and contact phone numbers 12 Pre-emptive prescribing The pre-emptive prescribing of p.r.n. medication for anticipated symptoms can avoid great distress. The cost is negligible and it saves time on the part of both families and out-of-hours health professionals. Typical maximum doses are described on page 22, but the doses needed by individuals vary widely: it is more important to assess the effectiveness of each p.r.n. before repeating or increasing doses – if a p.r.n. is ineffective, try a different approach or seek advice (see flow diagrams) A typical “pre-emptive p.r.n.” regimen for patients approaching the end of life might include: Morphine sulphate 2.5-5mg 1-4 hourly p.r.n. -

Methamphetamine Presentations to an Emergency Department: Management and Complications
Isoardi Katherine (Orcid ID: 0000-0002-1176-7923) Abstract Objective: There is little recent published data characterising methamphetamine intoxication. This study aims to describe the clinical effects, management, complications and disposition of patients with methamphetamine exposure. Methods: This is a retrospective review of patients presenting with methamphetamine intoxication to an Emergency Department in 2016. All presentations were extracted from a relational database and each medical record reviewed. Demographics, clinical features, complications and disposition were extracted. Results: There were 378 presentations of 329 patients (234 males [71%]), median age 31 years (range 16-68 years). The commonest clinical effect was acute behavioural disturbance, occurring in 295 (78%) presentations. This was successfully managed with oral sedation alone in 180 (61%) patients with the remainder receiving parenteral sedation. Other effects included tachycardia in 212 (56%), hypertension in 160 (42%) and hyperthermia in 17 (4%) presentations. No antihypertensives were given. One patient was actively cooled. Complications included 21 (30%) presentations with rhabdomyolysis and 43 (11%) presentations with acute kidney injury. There were two seizures, three intracranial bleeds and one myocardial infarction. The majority of This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/1742-6723.13219 This article is protected by copyright. All rights reserved. patients (310 [82%]) were managed solely within the emergency department. The median length of stay was 14 hours. There were 41(11%) mental health admissions. -
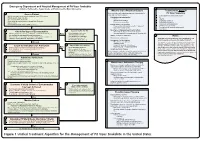
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

10Neurodevelopmental Effects of Childhood Exposure to Heavy
Neurodevelopmental E¤ects of Childhood Exposure to Heavy Metals: 10 Lessons from Pediatric Lead Poisoning Theodore I. Lidsky, Agnes T. Heaney, Jay S. Schneider, and John F. Rosen Increasing industrialization has led to increased exposure to neurotoxic metals. By far the most heavily studied of these metals is lead, a neurotoxin that is particularly dangerous to the developing nervous system of children. Awareness that lead poison- ing poses a special risk for children dates back over 100 years, and there has been increasing research on the developmental e¤ects of this poison over the past 60 years. Despite this research and growing public awareness of the dangers of lead to chil- dren, government regulation has lagged scientific knowledge; legislation has been in- e¤ectual in critical areas, and many new cases of poisoning occur each year. Lead, however, is not the only neurotoxic metal that presents a danger to children. Several other heavy metals, such as mercury and manganese, are also neurotoxic, have adverse e¤ects on the developing brain, and can be encountered by children. Al- though these other neurotoxic metals have not been as heavily studied as lead, there has been important research describing their e¤ects on the brain. The purpose of the present chapter is to review the neurotoxicology of lead poisoning as well as what is known concerning the neurtoxicology of mercury and manganese. The purpose of this review is to provide information that might be of some help in avoiding repeti- tion of the mistakes that were made in attempting to protect children from the dan- gers of lead poisoning. -

Unintentional Fentanyl Overdoses Among Persons Who Thought They
Morbidity and Mortality Weekly Report Notes from the Field Unintentional Fentanyl Overdoses Among emergency departments; 2) holding a multiagency press Persons Who Thought They Were Snorting conference; 3) conducting media interviews; 4) informing Cocaine — Fresno, California, January 7, 2019 law enforcement, prehospital providers, and the public about Patil Armenian, MD1; Jeffrey D. Whitman, MD2; Adina Badea, PhD2; naloxone distribution and use; 5) educating persons on Whitney Johnson, MD1; Chelsea Drake, MS1; Simranjit Singh Dhillon3; the proper disposal of old or new but unused medications Michelle Rivera3; Nicklaus Brandehoff, MD1; Kara L. Lynch, PhD2 through the Fresno County Department of Behavioral Health/ California Health Collaborative drop-off containers§; and On January 7, 2019, three patients arrived at the Community 6) publicizing the California Central Valley Opioid Safety Regional Medical Center emergency department in Fresno, Coalition webpage,¶ which provides information about California, after snorting (i.e., nasally insufflating) white pow- naloxone and substance use disorders. der they thought was cocaine. One (patient A) was in cardiac On January 12, 2019, a similar drug overdose incident was arrest, and two (patients B and C) had opioid toxidrome reported in Chico, California, in which postmortem toxicol- (miosis, respiratory depression, and depressed mental status) ogy testing for one person confirmed fentanyl (1). Fourteen (Table). After spontaneous circulation was reestablished in other persons at the same event were hospitalized with opioid patient A, he was admitted to the intensive care unit, where toxidrome and later released. They reported thinking they he was pronounced brain-dead 3 days later. Patients B and C were snorting cocaine,** but confirmatory toxicology results responded to naloxone, but repeated dosing was required to are unavailable. -

Poison Prevention Packaging: a Guide for Healthcare Professionals
PPooiissoonn PPrreevveennttiioonn PPaacckkaaggiinngg:: AA GGuuiiddee FFoorr HHeeaalltthhccaarree PPrrooffeessssiioonnaallss REVISED 2005 CPSC 384 US. CONSUMER PRODUCT SAFETY COMMISSION, WASHINGTON, D.C. 20207 THIS BROCHURE BROUGHT TO YOU BY: U.S. CONSUMER PRODUCT SAFETY COMMISSION Washington, DC 20207 Web site: www.cpsc.gov Toll-free hotline: 1-800-638-2772 The U.S. Consumer Product Safety Commission (CPSC) is a federal agency that helps keep families and children safe in and around their homes. For more information, call the CPSC’s toll-free hotline at 1-800-638-2772 or visit its website at http://www.cpsc.gov. Poison Prevention Packaging: A Guide For Healthcare Professionals (revised 2005) Preface The U.S. Consumer Product Safety Commission (CPSC) administers the Poison Prevention Packaging Act of 1970 (PPPA), 15 U.S.C. §§ 1471-1476. The PPPA requires special (child-resistant and adult-friendly) packaging of a wide range of hazardous household products including most oral prescription drugs. Healthcare professionals are more directly involved with the regulations dealing with drug products than household chemical products. Over the years that the regulations have been in effect, there have been remarkable declines in reported deaths from ingestions by children of toxic household substances including medications. Despite this reduction in deaths, many children are poisoned or have "near-misses" with medicines and household chemicals each year. Annually, there are about 30 deaths of children under 5 years of age who are unintentionally poisoned. Data from the National Electronic Injury Surveillance System (a CPSC database of emergency room visits) indicate that in 2003, an estimated 78,000 children under 5 years of age were treated for poisonings in hospital emergency rooms in the United States. -

Future Directions for Intrathecal Pain Management 93
NEUROMODULATION: TECHNOLOGY AT THE NEURAL INTERFACE Volume 11 • Number 2 • 2008 http://www.blackwell-synergy.com/loi/ner ORIGINAL ARTICLE FBlackwell uturePublishing Inc Directions for Intrathecal Pain Management: A Review and Update From the Interdisciplinary Polyanalgesic Consensus Conference 2007 Timothy Deer, MD* • Elliot S. Krames, MD† • Samuel Hassenbusch, MD, PhD‡ • Allen Burton, MD§ • David Caraway, MD¶ • Stuart Dupen, MD** • James Eisenach, MD†† • Michael Erdek, MD‡‡ • Eric Grigsby, MD§§ • Phillip Kim, MD¶¶ • Robert Levy, MD, PhD*** • Gladstone McDowell, MD††† • Nagy Mekhail, MD‡‡‡ • Sunil Panchal, MD§§§ • Joshua Prager, MD¶¶¶ • Richard Rauck, MD**** • Michael Saulino, MD†††† •Todd Sitzman, MD‡‡‡‡ • Peter Staats, MD§§§§ • Michael Stanton-Hicks, MD¶¶¶¶ • Lisa Stearns, MD***** • K. Dean Willis, MD††††† • William Witt, MD‡‡‡‡‡ • Kenneth Follett, MD, PhD§§§§§ • Mark Huntoon, MD¶¶¶¶¶ • Leong Liem, MD****** • James Rathmell, MD†††††† • Mark Wallace, MD‡‡‡‡‡‡ • Eric Buchser, MD§§§§§§ • Michael Cousins, MD¶¶¶¶¶¶ • Ann Ver Donck, MD******* *Charleston, WV; †San Francisco, CA; ‡Houston, TX; §Houston, TX; ¶Huntington, WV; **Bellevue, WA; ††Winston Salem, NC; ‡‡Baltimore, MD; §§Napa, CA; ¶¶Wilmington, DE; ***Chicago, IL; †††Columbus, OH; ‡‡‡Cleveland, OH; §§§Tampa, FL; ¶¶¶Los Angeles, CA; ****Winston Salem, NC; ††††Elkings Park, PA; ‡‡‡‡Hattiesburg, MS; §§§§Colts Neck, NJ; ¶¶¶¶Cleveland, OH; *****Scottsdale, AZ; †††††Huntsville, AL; ‡‡‡‡‡Lexington, KY; §§§§§Iowa City, IA; ¶¶¶¶¶Rochester, NY; ******Nieuwegein, The Netherlands; ††††††Boston, MA; ‡‡‡‡‡‡La Jolla, CA; §§§§§§Switzerland; ¶¶¶¶¶¶Australia; and *******Brugge, Belgium ABSTRACT Background. Expert panels of physicians and nonphysicians, all expert in intrathecal (IT) therapies, convened in the years 2000 and 2003 to make recommendations for the rational use of IT analgesics, based on the preclinical and clinical literature known up to those times, presentations of the expert panels, discussions on current practice and standards, and the result of surveys of physicians using IT agents. -

Medical Toxicology Milestone Project
The Medical Toxicology Milestone Project A Joint Initiative of The Accreditation Council for Graduate Medical Education and The American Board of Emergency Medicine July 2015 The Medical Toxicology Milestone Project The Milestones are designed only for use in evaluation of the fellow in the context of their participation in ACGME-accredited residency or fellowship programs. The Milestones provide a framework for assessment of the development of the fellow in key dimensions of the elements of physician competency in a specialty or subspecialty. They neither represent the entirety of the dimensions of the six domains of physician competency, nor are they designed to be relevant in any other context. i Medical Toxicology Milestones Chair: Susanne White, MD Working Group Advisory Group Michele M. Burns, MD, MPH Timothy Brigham, MDiv, PhD Beth Baker, MD Wallace Carter, MD Laura Edgar, EdD, CAE William W. Greaves, MD, MSPH Lewis Nelson, MD Robert Johnson, MD Louis Ling, MD Earl Reisdorff, MD ii Milestone Reporting This document presents milestones designed for programs to use in semi-annual review of fellow performance and reporting to the ACGME. Milestones are knowledge, skills, attitudes, and other attributes for each of the ACGME competencies organized in a developmental framework from less to more advanced. They are descriptors and targets for fellow performance as a fellow moves from entry into fellowship through graduation. In the initial years of implementation, the Review Committee will examine milestone performance data for each program’s fellows as one element in the Next Accreditation System (NAS) to determine whether fellows overall are progressing. For each period, review and reporting will involve selecting milestone levels that best describe a fellow’s current performance and attributes. -

Hyoscine Butylbromide, Levomepromazine, Metoclopramide, Midazolam, Ondansetron
TRUST WIDE/DIVISIONAL DOCUMENT Delete as appropriate Policy/Standard Operating Procedure/ Clinical Guideline Policy and Procedure for the T34 Ambulatory Syringe Pump DOCUMENT TITLE: in adults (Palliative Care) DOCUMENT ELHT/CP22 Version 5.3 NUMBER: DOCUMENT REPLACES Which ELHT/CP22 Version 5.2 Version LEAD EXECUTIVE DIRECTOR DGM AUTHOR(S): Note Syringe pump policy task and finish group chaired by should not include Palliative Medicine Consultant names TARGET AUDIENCE: Medical and Nursing Staff 1 To provide a clear governance framework to ensure a safe and consistent approach to the use of the T34 Ambulatory Syringe Pump DOCUMENT 2 To provide details of how to set up and administer PURPOSE: medication by a T34 Ambulatory Syringe Pump 3 To provide easily accessible information about the common medicines used in a Syringe Pump Clinical Practice Summary. Guidance on consensus approaches To be read in to managing palliative care symptoms. Lancashire and South conjunction with Cumbria consensus guidance – August 2017 (identify which internal C064 V5 Medicines Management Policy documents) IC24 V4 Aseptic non touch technique (ANTT) policy East Lancashire Hospital NHS Trust – Policies & Procedures, Protocols, Guidelines ELHT/CP22 v5.2 May 2020 Page 1 of 77 Nursing and Midwifery Council - Standards for Medicines Management 2015 Dickman et al (2016) The Syringe Driver, 4th edition, Oxford Press T. Mitten, (2000) Subcutaneous drug infusions, a review of problems and solutions. International Journal of Palliative Nursing Vol 7, No 2. Twycross et al (2017) 6th Edition Palliative Care SUPPORTING Formulary and Palliative Care Formulary online, REFERENCES accessed June 2018 Twycross R., Wilcock A., (2001) Symptom Management in Advanced Cancer 3rd edition Radcliffe medical press Oxon. -

Methadone Poisonings in France
2019 Extended Abstract Clinical Pharmacology & Toxicology Journal Vol. 3 No.2 Methadone Poisonings in France: A Seven-Year Experience of the French Poison Control Center Network Katharina von Fabeck1, Romain Torrents1, 2, Mathieu Glaizal1, Luc de Haro1, Nicolas Simon 1 Centre Antipoison et de Toxicovigilance, Service de Pharmacologie Clinique, Hôpital Sainte Marguerite, APHM, Marseille, France 2 Aix-Marseille University, Marseille, France Methadone is an opioid agonist prescribed in France for the methadone must be considered as a highly toxic medicine. treatment of opioid dependency. In order to evaluate the This investigation is a review examination of methadone exposures clinical toxicity of methadone, the authors present a seven- answered to the nine FPCC between October 15, 2010 and October year experience of the French Poison Control Center (FPCC) 15, 2017. The two pharmaceutical structures (case and syrup) Network at the national level. This study is a retrospective were thought of. Youth inadvertent poisonings were prohibited analysis of methadone exposures reported to the nine FPCC (diverse examination). 1415 instances of methadone harming between October 15th 2010 and October 15th 2017. The two were incorporated (29% female, 71% male, normal age 34 +/ - pharmaceutical forms (capsule and syrup) were considered. 10). 90% of the patients had history of compulsion and 69% were Childhood accidental poisonings were excluded (different treated with methadone (31% were gullible patients). The two study). 1415 cases of methadone poisoning were included (29% primary conditions were addictions (47% of the cases) and self- female, 71% male, average age 34 +/-10). 90% of the patients destruction endeavors (41%). In 45% of the cases it was containers, had history of addiction and 69% were treated with methadone in 35% syrup, obscure for 20%.