National Licensed Medicine List of Afghanistan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Snake Bite Protocol
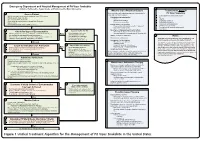
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Specifications of Approved Drug Compound Library
Annexure-I : Specifications of Approved drug compound library The compounds should be structurally diverse, medicinally active, and cell permeable Compounds should have rich documentation with structure, Target, Activity and IC50 should be known Compounds which are supplied should have been validated by NMR and HPLC to ensure high purity Each compound should be supplied as 10mM solution in DMSO and at least 100µl of each compound should be supplied. Compounds should be supplied in screw capped vial arranged as 96 well plate format. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Evaluating Disinfectants for Use Against the COVID-19 Virus
When it comes to choosing a disinfectant to combat the COVID-19 virus, research and health authorities suggest not all disinfectants are equally effective. The difference is in their active ingredient(s). HEALTH CANADA AND U.S. EPA ASSESSMENTS The work to evaluate disinfectants perhaps best starts with lists of approved disinfectants compiled by government health authorities. Health Canada has compiled a list of 85 hard surface disinfectant products (as of March 20, 2020) that meet their requirements for disinfection of emerging pathogens, including the virus that causes COVID-19. It can be accessed here. You can wade through the entire list. But if you locate the Drug Identification Number (DIN) on the disinfectant product label or the safety data sheet (SDS), then you can use the search function to quickly see if the product meets Health Canada requirements. A second list, updated on March 19, 2020, provides 287 products that meet the U.S. Environmental Protection Agency’s (EPA) criteria for use against SARS-CoV-2, the novel coronavirus that causes the disease COVID-19. This list can be found here. Like the Health Canada list, you can wade through this one too. However, to best use this list, you should locate the U.S. EPA registration number on the product label or SDS, and use that number to search the list. The U.S. EPA registration number of a product consists of two sets of numbers separated by a hyphen. The first set of numbers refers to the company identification number, and the second set of numbers following the hyphen represents the product number. -

Tonsillopharyngitis - Acute (1 of 10)
Tonsillopharyngitis - Acute (1 of 10) 1 Patient presents w/ sore throat 2 EVALUATION Yes EXPERT Are there signs of REFERRAL complication? No 3 4 EVALUATION Is Group A Beta-hemolytic Yes DIAGNOSIS Streptococcus (GABHS) • Rapid antigen detection test infection suspected? (RADT) • roat culture No TREATMENT EVALUATION No A Supportive management Is GABHS confi rmed? B Pharmacological therapy (Non-GABHS) Yes 5 TREATMENT A EVALUATE RESPONSEMIMS Supportive management TO THERAPY C Pharmacological therapy • Antibiotics Poor/No Good D Surgery, if recurrent or complicated response response REASSESS PATIENT COMPLETE THERAPY & REVIEW THE DIAGNOSIS© Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B269 © MIMS Pediatrics 2020 Tonsillopharyngitis - Acute (2 of 10) 1 ACUTE TONSILLOPHARYNGITIS • Infl ammation of the tonsils & pharynx • Etiologies include bacterial (group A β-hemolytic streptococcus, Haemophilus infl uenzae, Fusobacterium sp, etc) & viral (infl uenza, adenovirus, coronavirus, rhinovirus, etc) pathogens • Sore throat is the most common presenting symptom in older children TONSILLOPHARYNGITIS 2 EVALUATION FOR COMPLICATIONS • Patients w/ sore throat may have deep neck infections including epiglottitis, peritonsillar or retropharyngeal abscess • Examine for signs of upper airway obstruction Signs & Symptoms of Sore roat w/ Complications • Trismus • Inability to swallow liquids • Increased salivation or drooling • Peritonsillar edema • Deviation of uvula -

FDA-2015-N-0101; and FDA-2016-N-0124
DE PARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 November 18, 2020 Docket Nos. FDA-1975-N-0012; FDA-2015-N-0101; and FDA-2016-N-0124 The American Cleaning Institute Attention: James Kim, PhD Vice President, Science and Regulatory Affairs 1401 H Street, N.W. Suite 700 Washington, D.C. 20005 Re: Benzalkonium Chloride, Benzethonium Chloride, Chloroxylenol, Ethanol, and Povidone-Iodine Dear Dr. Kim: This letter responds to The American Cleaning Institute’s (ACI’s) July 14, 2020 communication regarding the deferral from final rulemaking under the over-the-counter (OTC) Drug Review on benzalkonium chloride, benzethonium chloride, chloroxylenol, ethanol, and povidone-iodine for use in nonprescription (often referred to as over-the-counter or OTC) consumer antiseptic wash, health care antiseptic, and consumer antiseptic rub drug products. In March 2016, FDA issued letters granting requests to defer three active ingredients— benzalkonium chloride, benzethonium chloride, and chloroxylenol—from inclusion in the final rulemaking for the December 2013 proposed rule for OTC consumer antiseptic washes (78 FR 76444). Similarly, in January 2017, FDA issued letters granting requests to defer six active ingredients—benzalkonium chloride, benzethonium chloride, chloroxylenol, ethanol, povidone- iodine, and isopropyl alcohol—from inclusion in the final rulemaking for the May 2015 proposed rule for OTC health care antiseptics (80 FR 25166). In October 2017, FDA issued letters granting requests to defer three active -

Teriparatide Injection I.P. (R-DNA Origin) Interactions Were Noted
PA011FSRI04 For more information, visit us at www.lillyindia.co.in Interactions with other medicinal products and other forms of interaction Forteo® has been evaluated in pharmacodynamic interaction studies with hydrochlorothiazide. No clinically significant Teriparatide Injection I.P. (r-DNA origin) interactions were noted. ® Co-administration of raloxifene or hormone replacement therapy with Forteo® did not alter the effects of Forteo® on Forteo serum or urine calcium or on clinical adverse events. (600mcg/2.4mL, Solution for injection in a pre-filled pen) In a study of 15 healthy subjects administered digoxin daily to steady state, a single Forteo® dose did not alter the cardiac effect of digoxin. However, sporadic case reports have suggested that hypercalcaemia may predispose patients to digitalis toxicity. Because Forteo® transiently increases serum calcium, Forteo® should be used with caution in patients taking digitalis NAME OF THE MEDICINAL PRODUCT Fertility, pregnancy and lactation Fertility Forteo® 600mcg/2.4mL, solution for injection, in pre-filled pen. Studies in rabbits have shown reproductive toxicity (see section Preclinical safety data). The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. QUALITATIVE AND QUANTITATIVE COMPOSITION Pregnancy Each mL of Teriparatide Injection I.P. contains: 250 mcg Teriparatide I.P. (r-DNA origin) as active ingredient, Forteo® is contraindicated for use during pregnancy (see section Contraindications) 0.41 mg Glacial Acetic Acid I.P. as buffering agent, 0.10 mg Sodium Acetate (Anhydrous) I.P. as buffering agent, Breast –Feeding 45.4 mg Mannitol I.P. as tonicity modifier, 3.0 mg Metacresol Ph. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Chewable Lozenge Formulation
Umashankar M S et al. Int. Res. J. Pharm. 2016, 7 (4) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Review Article CHEWABLE LOZENGE FORMULATION- A REVIEW Umashankar M S *, Dinesh S R, Rini R, Lakshmi K S, Damodharan N SRM College of Pharmacy, SRM University, Kattankulathur, India *Corresponding Author Email: [email protected] Article Received on: 11/02/16 Revised on: 13/03/16 Approved for publication: 28/03/16 DOI: 10.7897/2230-8407.07432 ABSTRACT Development of lozenges dated back to 20thcentury and is still remain popular among the consumer and hence it has continued commercial production. Lozenges are palatable solid unit dosage form administrated in the oral cavity. They meant to be dissolved in mouth or pharynx for its local or systemic effect. Lozenge tablets provide several advantages as pharmaceutical formulations however with some disadvantages. Lozenge as a dosage form can be adopted for drug delivery across buccal route, labial route, gingival route and sublingual route. Multiple drugs can also be incorporated in them for chronic illness treatments. Lozenge enables loading of wide range of active ingredients for oral systemic delivery of drugs. Lozenges are available as over the counter medications in the form of caramel based soft lozenges, hard candy lozenges and compressed tablet lozenges containing drugs for sore throat, mouth infection and as mouth fresheners. The rationale behind the use of medicated lozenges as one of the most favored dosage form for the delivery of antitussive drugs. This review focuses various aspects of lozenge formulation providing an insight to the formulation scientist on novel application of lozenge drug delivery system. -

Substantial Equivalence Determination Decision Summary Assay Only Template
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k091024 B. Purpose for Submission: Premarket notification C. Measurand: Methicillin Resistant Staphylococcus aureus (MRSA) D. Type of Test: Detection of MRSA using a selective and differential chromogenic media E. Applicant: bioMérieux, Inc. F. Proprietary and Established Names: chromID™ MRSA Agar G. Regulatory Information: 1. Regulation section: 21 CFR 866.1700 2. Classification: Class II 3. Product code: JSO Culture media, Antimicrobial susceptibility test, excluding Mueller Hinton Agar 1 4. Panel: Microbiology H. Intended Use: 1. Intended use(s): chromID™ MRSA agar is a selective and differential chromogenic medium for the qualitative detection of nasal colonization of methicillin resistant Staphylococcus aureus (MRSA) to aid in the prevention and control of MRSA infections in healthcare settings. The test is performed on anterior nares swab specimens from patients and healthcare workers to screen for MRSA colonization. chromID™ MRSA agar is not intended to diagnose MRSA infection nor to guide or monitor treatment of infection. 2. Indication(s) for use: The chromID™ MRSA agar is not intended to diagnose MRSA infection nor to guide or monitor treatment of infection. 3. Special conditions for use statement(s): Prescription use 4. Special instrument requirements: Not applicable I. Device Description: The chromID™ MRSA agar is translucent and a light tan color. After the plates are inoculated and incubated, colonies growing on the plates will have either a green appearance, which indicates a positive MRSA status or a colorless appearance which indicates a negative MRSA status. The green color is more vivid if the colonies are observed through the agar from the bottom of the plate. -

Long-Term Effects of Snake Envenoming
toxins Review Long-Term Effects of Snake Envenoming Subodha Waiddyanatha 1,2, Anjana Silva 1,2 , Sisira Siribaddana 1 and Geoffrey K. Isbister 2,3,* 1 Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka, Saliyapura 50008, Sri Lanka; [email protected] (S.W.); [email protected] (A.S.); [email protected] (S.S.) 2 South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, University of Peradeniya, Peradeniya 20400, Sri Lanka 3 Clinical Toxicology Research Group, University of Newcastle, Callaghan, NSW 2308, Australia * Correspondence: [email protected] or [email protected]; Tel.: +612-4921-1211 Received: 14 March 2019; Accepted: 29 March 2019; Published: 31 March 2019 Abstract: Long-term effects of envenoming compromise the quality of life of the survivors of snakebite. We searched MEDLINE (from 1946) and EMBASE (from 1947) until October 2018 for clinical literature on the long-term effects of snake envenoming using different combinations of search terms. We classified conditions that last or appear more than six weeks following envenoming as long term or delayed effects of envenoming. Of 257 records identified, 51 articles describe the long-term effects of snake envenoming and were reviewed. Disability due to amputations, deformities, contracture formation, and chronic ulceration, rarely with malignant change, have resulted from local necrosis due to bites mainly from African and Asian cobras, and Central and South American Pit-vipers. Progression of acute kidney injury into chronic renal failure in Russell’s viper bites has been reported in several studies from India and Sri Lanka. Neuromuscular toxicity does not appear to result in long-term effects. -

Infektionskongress 11. Österreichischer
11. ÖSTERREICHISCHER INFEKTIONSKONGRESS WAS SIE IMMER SCHON WISSEN WOLLTEN Diagnostik – Therapie – Prophylaxe 29. März bis 1. April 2017 Hotel Gut Brandlhof, Saalfelden Information & Anmeldung: www.oegit.eu PROGRAMM PROGRAMM PROGRAMM VORWORT Unser ganz besonderer Dank gilt natürlich auch heuer wieder unseren Spon soren, die uns trotz schwieriger Zeiten im Gesundheitswesen die Treue halten. Ohne deren nachhaltiges Engagement wäre dieser Kongress in der gewohnten Form nicht realisierbar. Heuer haben wir auch eine kleine technische Neuigkeit für Sie vorbereitet: Sie werden während der Vorträge im Plenarsaal über Ihr Mobiltelefon anonym Fragen an die Referenten stellen können. Näheres werden Sie vor Ort am Brandlhof erfahren. Unten finden Sie den dafür nötigen QRCode bzw. Link. Sehr geehrte Kollegin, sehr geehrter Kollege, Wir hoffen, Ihnen mit dem vorliegenden Programm spannende Tage im Brandl wir freuen uns, Sie im Namen des Vorstandes der Österreichischen Gesellschaft für hof bieten zu können, und freuen uns schon auf das persönliche Gespräch mit Infektionskrankheiten und Tropenmedizin am 11. Österreichischen Infektions Ihnen in den Pausen. kongress in Saalfelden begrüßen zu dürfen. Das heurige Kongressthema „Was Sie immer schon wissen wollten: Diagnostik – Therapie – Prophylaxe“ steht für die Diskussion offener infektiologischer Frage stellungen und lädt zu interessanten Vorträgen ein. Das infektiologische Potpourri ist breit und behandelt den sinnvollen Einsatz von Kombinations Mit kollegialen Grüßen Xcom.events/oeginfekt therapien, ungewöhnliche Erkrankungen bei Asylsuchenden & Migranten sowie Impfungen – um nur ein paar Highlights des Kongresses zu nennen. Es ist uns auch heuer wieder gelungen, namhafte ReferentInnen zu wichtigen Themen zu gewinnen. Herr Professor Lemmen aus Aachen wird den Eröffnungs Univ.Prof. Dr. Cornelia LassFlörl Univ.Prof.