Redalyc.Use of Hen Egg Derived Immunoglobulin Against Scolopendra
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
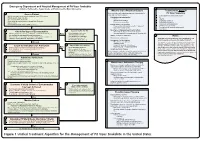
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Volume 4 Issue 1B
Captive & Field Herpetology Volume 4 Issue 1 2020 Volume 4 Issue 1 2020 ISSN - 2515-5725 Published by Captive & Field Herpetology Captive & Field Herpetology Volume 4 Issue1 2020 The Captive and Field Herpetological journal is an open access peer-reviewed online journal which aims to better understand herpetology by publishing observational notes both in and ex-situ. Natural history notes, breeding observations, husbandry notes and literature reviews are all examples of the articles featured within C&F Herpetological journals. Each issue will feature literature or book reviews in an effort to resurface past literature and ignite new research ideas. For upcoming issues we are particularly interested in [but also accept other] articles demonstrating: • Conflict and interactions between herpetofauna and humans, specifically venomous snakes • Herpetofauna behaviour in human-disturbed habitats • Unusual behaviour of captive animals • Predator - prey interactions • Species range expansions • Species documented in new locations • Field reports • Literature reviews of books and scientific literature For submission guidelines visit: www.captiveandfieldherpetology.com Or contact us via: [email protected] Front cover image: Timon lepidus, Portugal 2019, John Benjamin Owens Captive & Field Herpetology Volume 4 Issue1 2020 Editorial Team Editor John Benjamin Owens Bangor University [email protected] [email protected] Reviewers Dr James Hicks Berkshire College of Agriculture [email protected] JP Dunbar -

Review of the Subspecies of Scolopendra Subspinipes Leach, 1815 with the New Description of the South Chinese Member of the Genu
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Spixiana, Zeitschrift für Zoologie Jahr/Year: 2012 Band/Volume: 035 Autor(en)/Author(s): Kronmüller Christian Artikel/Article: Review of the subspecies of Scolopendra subspinipes Leach, 1815 with the new description of the South Chinese member of the genus Scolopendra Linnaeus, 1758 named Scolopendra hainanum spec. nov. (Myriapoda, Chilopoda, Scolopendridae). 19-27 ©Zoologische Staatssammlung München/Verlag Friedrich Pfeil; download www.pfeil-verlag.de SPIXIANA 35 1 19-27 München, August 2012 ISSN 0341-8391 Review of the subspecies of Scolopendra subspinipes Leach, 1815 with the new description of the South Chinese member of the genus Scolopendra Linnaeus, 1758 named Scolopendra hainanum spec. nov. (Myriapoda, Chilopoda, Scolopendridae) Christian Kronmüller Kronmüller, C. 2012. Review of the subspecies of Scolopendra subspinipes Leach, 1815 with the new description of the South Chinese member of the genus Scolo- pendra Linnaeus, 1758 named Scolopendra hainanum spec. nov. (Myriapoda, Chilo- poda, Scolopendridae). Spixiana 35 (1): 19-27. To clarify their discrimination, the taxa of the Scolopendra subspinipes group, formerly treated as subspecies of this species, are reviewed. Scolopendra dehaani stat. revalid. and Scolopendra japonica stat. revalid. are reconfirmed at species level. Scolopendra subspinipes cingulatoides is raised to species level. This species is re- named to Scolopendra dawydoffi nom. nov. to avoid homonymy with Scolopendra cingulatoides Newport, 1844 which was placed in synonymy under Scolopendra cingulata Latreille, 1829 by Kohlrausch (1881). Scolopendra subspinipes piceoflava syn. nov. and Scolopendra subspinipes fulgurans syn. nov. are proposed as new synonyms of Scolopendra subspinipes, which is now without subspecies. -

Linnaeus at Home
NATURE-BASED ACTIVITIES FOR PARENTS LINNAEUS 1 AT HOME A GuiDE TO EXPLORING NATURE WITH CHILDREN Acknowledgements Written by Joe Burton Inspired by Carl Linnaeus With thanks to editors and reviewers: LINNAEUS Lyn Baber, Melissa Balzano, Jane Banham, Sarah Black, Isabelle Charmantier, Mark Chase, Maarten Christenhusz, Alex Davey, Gareth Dauley, AT HOME Zia Forrai, Jon Hale, Simon Hiscock, Alice ter Meulen, Lynn Parker, Elizabeth Rollinson, James Rosindell, Daryl Stenvoll-Wells, Ross Ziegelmeier Share your explorations @LinneanLearning #LinnaeusAtHome Facing page: Carl Linnaeus paper doll, illustrated in 1953. © Linnean Society of London 2019 All rights reserved. No part of this publication may be reproduced, stored in a retrival system or trasmitted in any form or by any means without the prior consent of the copyright owner. www.linnean.org/learning “If you do not know Introduction the names of things, the knowledge of them is Who was Carl Linnaeus? Contents Pitfall traps 5 lost too” Carl Linnaeus was one of the most influential scientists in the world, - Carl Linnaeus A bust of ‘The Young Linnaeus’ by but you might not know a lot about him. Thanks to Linnaeus, we Bug hunting 9 Anthony Smith (2007). have a naming system for all species so that we can understand how different species are related and can start to learn about the origins Plant hunting 13 of life on Earth. Pond dipping 17 As a young man, Linnaeus would study the animals, plants, Bird feeders 21 minerals and habitats around him. By watching the natural world, he began to understand that all living things are adapted to their Squirrel feeders 25 environments and that they can be grouped together by their characteristics (like animals with backbones, or plants that produce Friendly spaces 29 spores). -

Louisiana's Animal Species of Greatest Conservation Need (SGCN)
Louisiana's Animal Species of Greatest Conservation Need (SGCN) ‐ Rare, Threatened, and Endangered Animals ‐ 2020 MOLLUSKS Common Name Scientific Name G‐Rank S‐Rank Federal Status State Status Mucket Actinonaias ligamentina G5 S1 Rayed Creekshell Anodontoides radiatus G3 S2 Western Fanshell Cyprogenia aberti G2G3Q SH Butterfly Ellipsaria lineolata G4G5 S1 Elephant‐ear Elliptio crassidens G5 S3 Spike Elliptio dilatata G5 S2S3 Texas Pigtoe Fusconaia askewi G2G3 S3 Ebonyshell Fusconaia ebena G4G5 S3 Round Pearlshell Glebula rotundata G4G5 S4 Pink Mucket Lampsilis abrupta G2 S1 Endangered Endangered Plain Pocketbook Lampsilis cardium G5 S1 Southern Pocketbook Lampsilis ornata G5 S3 Sandbank Pocketbook Lampsilis satura G2 S2 Fatmucket Lampsilis siliquoidea G5 S2 White Heelsplitter Lasmigona complanata G5 S1 Black Sandshell Ligumia recta G4G5 S1 Louisiana Pearlshell Margaritifera hembeli G1 S1 Threatened Threatened Southern Hickorynut Obovaria jacksoniana G2 S1S2 Hickorynut Obovaria olivaria G4 S1 Alabama Hickorynut Obovaria unicolor G3 S1 Mississippi Pigtoe Pleurobema beadleianum G3 S2 Louisiana Pigtoe Pleurobema riddellii G1G2 S1S2 Pyramid Pigtoe Pleurobema rubrum G2G3 S2 Texas Heelsplitter Potamilus amphichaenus G1G2 SH Fat Pocketbook Potamilus capax G2 S1 Endangered Endangered Inflated Heelsplitter Potamilus inflatus G1G2Q S1 Threatened Threatened Ouachita Kidneyshell Ptychobranchus occidentalis G3G4 S1 Rabbitsfoot Quadrula cylindrica G3G4 S1 Threatened Threatened Monkeyface Quadrula metanevra G4 S1 Southern Creekmussel Strophitus subvexus -

Exposure of Humans Or Animals to Sars-Cov-2 from Wild, Livestock, Companion and Aquatic Animals Qualitative Exposure Assessment
ISSN 0254-6019 Exposure of humans or animals to SARS-CoV-2 from wild, livestock, companion and aquatic animals Qualitative exposure assessment FAO ANIMAL PRODUCTION AND HEALTH / PAPER 181 FAO ANIMAL PRODUCTION AND HEALTH / PAPER 181 Exposure of humans or animals to SARS-CoV-2 from wild, livestock, companion and aquatic animals Qualitative exposure assessment Authors Ihab El Masry, Sophie von Dobschuetz, Ludovic Plee, Fairouz Larfaoui, Zhen Yang, Junxia Song, Wantanee Kalpravidh, Keith Sumption Food and Agriculture Organization for the United Nations (FAO), Rome, Italy Dirk Pfeiffer City University of Hong Kong, Hong Kong SAR, China Sharon Calvin Canadian Food Inspection Agency (CFIA), Science Branch, Animal Health Risk Assessment Unit, Ottawa, Canada Helen Roberts Department for Environment, Food and Rural Affairs (Defra), Equines, Pets and New and Emerging Diseases, Exotic Disease Control Team, London, United Kingdom of Great Britain and Northern Ireland Alessio Lorusso Istituto Zooprofilattico dell’Abruzzo e Molise, Teramo, Italy Casey Barton-Behravesh Centers for Disease Control and Prevention (CDC), One Health Office, National Center for Emerging and Zoonotic Infectious Diseases, Atlanta, United States of America Zengren Zheng China Animal Health and Epidemiology Centre (CAHEC), China Animal Health Risk Analysis Commission, Qingdao City, China Food and Agriculture Organization of the United Nations Rome, 2020 Required citation: El Masry, I., von Dobschuetz, S., Plee, L., Larfaoui, F., Yang, Z., Song, J., Pfeiffer, D., Calvin, S., Roberts, H., Lorusso, A., Barton-Behravesh, C., Zheng, Z., Kalpravidh, W. & Sumption, K. 2020. Exposure of humans or animals to SARS-CoV-2 from wild, livestock, companion and aquatic animals: Qualitative exposure assessment. FAO animal production and health, Paper 181. -

Chilopoda; Scolopendridae
Bijdragen tot dl Dierkunde, 55 (1): 125-130 — 1985 Possible species isolation mechanisms in some scolopendrid centipedes (Chilopoda; Scolopendridae) by J.G.E. Lewis Taunton School, Taunton, Somerset TA2 6AD, England the femur of Abstract are seen on Eupolybothrus spp. Various other lithobiid secondary sexual sexual characters in dis- Secondary centipedes are briefly characters have been reviewed by Lewis (1981). cussed and it is that the the suggested spines on prefemora of the described Differences type above are of the last pair of legs in some scolopendrids are used in presumably associated with mating behaviour specific discrimination prior to mating. The hypothesis is in which male and female head discussed with reference of the come together to Scolopendra spp. eastern Mediterranean,north-east Africa and Arabia. to tail, and the antennae and the last pair of legs Where species of Scolopendra with identical The virtually are tapped. morphological adaptations in spinulation on the last legs are sympatric, a large size dif- the males would serve for the femalesto identify ference exists between them. different and sex. It is that in where the species suggested some genera prefemoral have been inhibited. In spines are absent, speciation may the where be absent genus Otostigmus spines may or spine similar in number of other patterns are very a species DIMORPHISM IN SCOLOPENDRIDAE secondary sexual characters have developed. Sexual is also in dimorphism seen the scolopen- INTRODUCTION dromorph family Scolopendridae. In Scolopendra morsitans Linnaeus the dorsal side of the Centipedes not infrequently exhibit secondary prefemur, femur and sometimes the tibiaof the last of is sexual characters. -

Morphology, Histology and Histochemistry of the Venom Apparatus of the Centipede, Scolopendra Valida (Chilopoda, Scolopendridae)
Int. J. Morphol., 28(1):19-25, 2010. Morphology, Histology and Histochemistry of the Venom Apparatus of the Centipede, Scolopendra valida (Chilopoda, Scolopendridae) Morfología, Histología e Histoquímica del Aparato Venenoso del Ciempiés, Scolopendra valida (Chilopoda, Scolopendridae) Bashir M. Jarrar JARRAR, B. M. Morphology, histology and histochemistry of the venom apparatus of the centipede, Scolopendra valida ( Chilopoda, Scolopendridae). Int. J. Morphol., 28(1):19-25, 2010. SUMMARY: Morphological, histological and histochemical characterizations of the venom apparatus of the centapede, S. valida have been investigated. The venom apparatus of Scolopendra valida consists of a pair of maxillipedes and venom glands situated anteriorly in the prosoma on either side of the first segment of the body. Each venom gland is continuous with a hollow tubular claw possessing a sharp tip and subterminal pore located on the outer curvature. The glandular epithelium is folded and consists of a mass of secretory epithelium, covered by a sheath of striated muscles. The secretory epithelium consists of high columnar venom-producing cells having dense cytoplasmic venom granules. The glandular canal lacks musculature and is lined with chitinous internal layer and simple cuboidal epithelium. The histochemical results indicate that the venom-producing cells of both glands elaborate glycosaminoglycan, acid mucosubstances, certain amino acids and proteins, but are devoid of glycogen. The structure and secretions of centipede venom glands are discussed within the context of the present results. KEY WORDS: Scolopendra valida; Venom apparatus; Microanatomy; Centapede; Saudi Arabia. INTRODUCTION Centipedes are distributed widely, especially in warm, centipedes have been reported to cause constitutional and temperate and tropical region (Norris, 1999; Lewis, 1981, systemic symptoms including: severe pain, local pruritus, 1996). -

Orb-Weaving Spider, Argiope Savignyi (Araneidae), Predation on the Proboscis Bat Rhynchonycteris Naso (Emballonuridae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by KU ScholarWorks 282 NOTES Caribbean Journal of Science, Vol. 43, No. 2, 282-284, 2007 Copyright 2007 College of Arts and Sciences University of Puerto Rico, Mayagu¨ ez Orb-weaving Spider, Argiope savignyi (Araneidae), Predation on the Proboscis Bat Rhynchonycteris naso (Emballonuridae) ROBERT M. TIMM1 AND MAURICIO LOSILLA2 1Natural History Museum and Biodiversity Re- search Center & Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, KS 66045-7561, USA; 2Escuela de Biología, Universidad de Costa Rica, Ciudad Universitaria, Costa Rica. Corresponding au- thor: [email protected] ABSTRACT.—We report an observation of an orb- weaving spider (Argiope savignyi; Araneidae) cap- turing and feeding on a proboscis bat (Rhynchonyc- teris naso; Emballonuridae) at the La Selva Biological Station in the Caribbean lowlands of Costa Rica. This observation and others suggest that spiders prey upon small bats more frequently than has been noted previously, and that invertebrates should now be considered as regular predators on small bats. KEYWORDS.—Argiope, Chiroptera, Costa Rica, preda- tion, Rhynchonycteris, sac-winged bat Bats are preyed upon by a wide range of vertebrate predators but there are few re- cords of invertebrate predators. Gillette and Kimbrough (1970) listed five inverte- brate groups as potential predators— American cockroaches (Pariplaneta america- nus), Australian cockroaches (Pariplaneta australis), driver ants, scorpions, and large spiders. Molinari et al. (2005) documented predation on three species of bats by giant centipedes (Scolopendra gigantea) in a Ven- ms. received Aug. 29, 2006; accepted April 23, 2007 NOTES 283 ezuelan cave; the centipedes killed and consumed adult bats that were captured while they roosted on the ceiling of the cave. -

Spider and Scorpion Case
Spider and Scorpion case Black widow spider (Lactrodectus hesperus) Black widows are notorious spiders identified by the colored, hourglass-shaped mark on their abdomens. Several species answer to the name, and they are found in temperate regions around the world. This spider's bite is much feared because its venom is reported to be 15 times stronger than a rattlesnake's. In humans, bites produce muscle aches, nausea, and a paralysis of the diaphragm that can make breathing difficult; however, contrary to popular belief, most people who are bitten suffer no serious damage—let alone death. But bites can be fatal—usually to small children, the elderly, or the infirm. Fortunately, fatalities are fairly rare; the spiders are nonaggressive and bite only in self-defense, such as when someone accidentally sits on them. These spiders spin large webs in which females suspend a cocoon with hundreds of eggs. Spiderlings disperse soon after they leave their eggs, but the web remains. Black widow spiders also use their webs to ensnare their prey, which consists of flies, mosquitoes, grasshoppers, beetles, and caterpillars. Black widows are comb- footed spiders, which means they have bristles on their hind legs that they use to cover their prey with silk once it has been trapped. To feed, black widows puncture their insect prey with their fangs and administer digestive enzymes to the corpses. By using these enzymes, and their gnashing fangs, the spiders liquefy their prey's bodies and suck up the resulting fluid. Giant desert hairy scorpion (Hadrurus arizonensis) Hadrurus arizonensis is distributed throughout the Sonora and Mojave deserts. -

Long-Term Effects of Snake Envenoming
toxins Review Long-Term Effects of Snake Envenoming Subodha Waiddyanatha 1,2, Anjana Silva 1,2 , Sisira Siribaddana 1 and Geoffrey K. Isbister 2,3,* 1 Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka, Saliyapura 50008, Sri Lanka; [email protected] (S.W.); [email protected] (A.S.); [email protected] (S.S.) 2 South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, University of Peradeniya, Peradeniya 20400, Sri Lanka 3 Clinical Toxicology Research Group, University of Newcastle, Callaghan, NSW 2308, Australia * Correspondence: [email protected] or [email protected]; Tel.: +612-4921-1211 Received: 14 March 2019; Accepted: 29 March 2019; Published: 31 March 2019 Abstract: Long-term effects of envenoming compromise the quality of life of the survivors of snakebite. We searched MEDLINE (from 1946) and EMBASE (from 1947) until October 2018 for clinical literature on the long-term effects of snake envenoming using different combinations of search terms. We classified conditions that last or appear more than six weeks following envenoming as long term or delayed effects of envenoming. Of 257 records identified, 51 articles describe the long-term effects of snake envenoming and were reviewed. Disability due to amputations, deformities, contracture formation, and chronic ulceration, rarely with malignant change, have resulted from local necrosis due to bites mainly from African and Asian cobras, and Central and South American Pit-vipers. Progression of acute kidney injury into chronic renal failure in Russell’s viper bites has been reported in several studies from India and Sri Lanka. Neuromuscular toxicity does not appear to result in long-term effects. -

Snakebite: the World's Biggest Hidden Health Crisis
Snakebite: The world's biggest hidden health crisis Snakebite is a potentially life-threatening neglected tropical disease (NTD) that is responsible for immense suffering among some 5.8 billion people who are at risk of encountering a venomous snake. The human cost of snakebite Snakebite Treatment Timeline Each year, approximately 5.4 million people are bitten by a snake, of whom 2.7 million are injected with venom. The first snake antivenom This leads to 400,000 people being permanently dis- produced, against the Indian Cobra. abled and between 83,000-138,000 deaths annually, Immunotherapy with animal- mostly in sub-Saharan Africa and South Asia. 1895 derived antivenom has continued to be the main treatment for snakebite evenoming for 120 years Snakebite: both a consequence and a cause of tropical poverty The Fav-Afrique antivenom, 2014 produced by Sanofi Pasteur (France) Survivors of untreated envenoming may be left with permanently discontinued amputation, blindness, mental health issues, and other forms of disability that severely affect their productivity. World Health Organization Most victims are agricultural workers and children in 2018 (WHO) lists snakebite envenoming the poorest parts of Africa and Asia. The economic as a neglected tropical disease cost of treating snakebite envenoming is unimaginable in most communities and puts families and communi- ties at risk of economic peril just to pay for treatment. WHO launches a strategy to prevent and control snakebite envenoming, including a program targeting affected communities and their health systems Global antivenom crisis 2019 The world produces less than half of the antivenom it The Scientific Research Partnership needs, and this only covers 57% of the world’s species for Neglected Tropical Snakbites of venomous snake.