Report Mapping of Charcot-Marie-Tooth Disease Type
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name epithelial membrane protein 2 Gene Symbol Emp2 Organism Rat Gene Summary Description Not Available Gene Aliases Not Available RefSeq Accession No. Not Available UniGene ID Rn.21730 Ensembl Gene ID ENSRNOG00000002664 Entrez Gene ID 360468 Assay Information Unique Assay ID qRnoCIP0024746 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENSRNOT00000003615 Amplicon Context Sequence CCTGCTGCCTTCGCTGCCCTGTGAACATGTTGGTGATTCTTGCCTTCATCATCGT CTTCCACATCGTGTCTACGGCACTCTTGTTCATTTCAACCATTGACAATGCCTGG TGGGTAGGAGATGGCTTCTCAGCT Amplicon Length (bp) 104 Chromosome Location 10:4276703-4281842 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 99 R2 0.9989 cDNA Cq 20.98 cDNA Tm (Celsius) 82.5 gDNA Cq Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report Emp2, Rat Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect. -

(12) United States Patent (10) Patent No.: US 7.873,482 B2 Stefanon Et Al
US007873482B2 (12) United States Patent (10) Patent No.: US 7.873,482 B2 Stefanon et al. (45) Date of Patent: Jan. 18, 2011 (54) DIAGNOSTIC SYSTEM FOR SELECTING 6,358,546 B1 3/2002 Bebiak et al. NUTRITION AND PHARMACOLOGICAL 6,493,641 B1 12/2002 Singh et al. PRODUCTS FOR ANIMALS 6,537,213 B2 3/2003 Dodds (76) Inventors: Bruno Stefanon, via Zilli, 51/A/3, Martignacco (IT) 33035: W. Jean Dodds, 938 Stanford St., Santa Monica, (Continued) CA (US) 90403 FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 WO WO99-67642 A2 12/1999 U.S.C. 154(b) by 158 days. (21)21) Appl. NoNo.: 12/316,8249 (Continued) (65) Prior Publication Data Swanson, et al., “Nutritional Genomics: Implication for Companion Animals'. The American Society for Nutritional Sciences, (2003).J. US 2010/O15301.6 A1 Jun. 17, 2010 Nutr. 133:3033-3040 (18 pages). (51) Int. Cl. (Continued) G06F 9/00 (2006.01) (52) U.S. Cl. ........................................................ 702/19 Primary Examiner—Edward Raymond (58) Field of Classification Search ................... 702/19 (74) Attorney, Agent, or Firm Greenberg Traurig, LLP 702/23, 182–185 See application file for complete search history. (57) ABSTRACT (56) References Cited An analysis of the profile of a non-human animal comprises: U.S. PATENT DOCUMENTS a) providing a genotypic database to the species of the non 3,995,019 A 1 1/1976 Jerome human animal Subject or a selected group of the species; b) 5,691,157 A 1 1/1997 Gong et al. -

Loss of Epithelial Membrane Protein-2 Expression Confers an Independent Prognosticator in Nasopharyngeal Carcinoma: a Cohort Study
Open Access Research BMJ Open: first published as 10.1136/bmjopen-2012-000900 on 5 April 2012. Downloaded from Loss of epithelial membrane protein-2 expression confers an independent prognosticator in nasopharyngeal carcinoma: a cohort study Yi-Hsien Chen,1 Li-Ching Wu,2 Wen-Ren Wu,3 Hung-Jung Lin,1 Sung-Wei Lee,4 Ching-Yih Lin,5 Shih-Lun Chang,6 Nan-Haw Chow,7,8 Hsuan-Ying Huang,9 Chien-Feng Li,2,3,10,11 Han-Ping Hsu,12 Yow-Ling Shiue3 To cite: Chen Y-H, Wu L-C, ABSTRACT ARTICLE SUMMARY Wu W-R, et al. Loss of Objective: To evaluate the expression of epithelial epithelial membrane membrane protein-2 (EMP2) protein and its Article focus protein-2 expression confers clinicopathological associations in patients with - an independent Recent studies have suggested that EMP2 plays nasopharyngeal carcinoma. prognosticator in a tumour suppressor role in B cell lymphomas. nasopharyngeal carcinoma: Design: Retrospective population-based cohort study. - Immunoexpression of EMP2 was retrospectively a cohort study. BMJ Open Setting: This study was based on a biobank in Chi-Mei assessed in biopsies of 124 consecutive patients 2012;2:e000900. Medical Center (Tainan, Taiwan) from 1993 to 2002. with nasopharyngeal carcinoma. doi:10.1136/ Participants: Biopsies of 124 consecutive bmjopen-2012-000900 nasopharyngeal carcinoma patients without initial Key messages - Loss of EMP2 expression significantly correlates distant metastasis and treated with consistent < Prepublication history for with advanced primary tumour, nodal status and guidelines were assessed. Immunoexpressions of this paper is available online. AJCC stage. To view these files please EMP2 were analysed and the outcomes were correlated - In multivariate analyses, loss of EMP2 expres- visit the journal online (http:// with clinicopathological features and patient survivals. -

Supplementary Data
Supplemental figures Supplemental figure 1: Tumor sample selection. A total of 98 thymic tumor specimens were stored in Memorial Sloan-Kettering Cancer Center tumor banks during the study period. 64 cases corresponded to previously untreated tumors, which were resected upfront after diagnosis. Adjuvant treatment was delivered in 7 patients (radiotherapy in 4 cases, cyclophosphamide- doxorubicin-vincristine (CAV) chemotherapy in 3 cases). 34 tumors were resected after induction treatment, consisting of chemotherapy in 16 patients (cyclophosphamide-doxorubicin- cisplatin (CAP) in 11 cases, cisplatin-etoposide (PE) in 3 cases, cisplatin-etoposide-ifosfamide (VIP) in 1 case, and cisplatin-docetaxel in 1 case), in radiotherapy (45 Gy) in 1 patient, and in sequential chemoradiation (CAP followed by a 45 Gy-radiotherapy) in 1 patient. Among these 34 patients, 6 received adjuvant radiotherapy. 1 Supplemental Figure 2: Amino acid alignments of KIT H697 in the human protein and related orthologs, using (A) the Homologene database (exons 14 and 15), and (B) the UCSC Genome Browser database (exon 14). Residue H697 is highlighted with red boxes. Both alignments indicate that residue H697 is highly conserved. 2 Supplemental Figure 3: Direct comparison of the genomic profiles of thymic squamous cell carcinomas (n=7) and lung primary squamous cell carcinomas (n=6). (A) Unsupervised clustering analysis. Gains are indicated in red, and losses in green, by genomic position along the 22 chromosomes. (B) Genomic profiles and recurrent copy number alterations in thymic carcinomas and lung squamous cell carcinomas. Gains are indicated in red, and losses in blue. 3 Supplemental Methods Mutational profiling The exonic regions of interest (NCBI Human Genome Build 36.1) were broken into amplicons of 500 bp or less, and specific primers were designed using Primer 3 (on the World Wide Web for general users and for biologist programmers (see Supplemental Table 2) [1]. -

The NIH Catalyst from the Deputy Director for Intramural Research
— Fostering Communication and Collaboration The nihCatalyst A Publication for NIH Intramural Scientists National Institutes of Health Office of the Director Volume 15 Issue 6 November-Dece.mber i , 2007 Research Festival Research Festival of Age: Getting to the Bottom Coming Tissue Engineering and Regenerative Medicine Of the Beta Cell by Fran Pollner by Julie Wallace or some, the quest is to increase the progenitor pool of pancre- anel chair Rocky F atic beta cells, to derive stem Tuan noted that cells that can be controlled in cul- P this was the NIH ture and serve as replacements for Research Festival’s damaged or lost beta cells; for oth- first dedicated sympo- ers, the quest is for new treatments. sium on tissue engi- neering and regenera- Stem Cell Studies tive medicine, a re- Typically, cul- flection that the field tured human beta is steadily approach- cells do not prolif- ing the threshold of erate well or retain clinical application. the mature pheno- Indeed, applying type, noted Marvin biological and engi- neering principles to Gershengorn, chief Fran Pollner The Re-Generation: (left to right): Pamela Robey, N1DCR; of the Clinical En- Marvin repairing and replac- Cynthia Dunbar, NHLB1; Catherine Kuo, NIAMS; and panel docrinology Branch Gershengorn ing damaged and de- chair Rocky Tuan, NIAMS and scientific director, NIDDK, who stroyed tissues has at- has been exploring the optimization tracted researchers across NIH; scientists adipocytes, and chondrocytes derived of hIPCs (human islet cell-derived from three institutes described their on- from MSCs could indeed be made to precursor cells) for about five years. -
Yale Computational Biology and Bioinformatics Rotation Guide 2020-2021
Yale Computational Biology and Bioinformatics Rotation Guide 2020-2021 Julien Berro ............................................................................................................................................................3 Cynthia Brandt .......................................................................................................................................................3 Kei-Hoi Cheung .....................................................................................................................................................4 Chris Cotsapas ........................................................................................................................................................4 Richard Flavell .......................................................................................................................................................4 Joel Gelernter .........................................................................................................................................................5 Mark Gerstein .........................................................................................................................................................6 Antonio Giraldez ....................................................................................................................................................7 Jeffrey Gruen ..........................................................................................................................................................8 -

Content Based Search in Gene Expression Databases and a Meta-Analysis of Host Responses to Infection
Content Based Search in Gene Expression Databases and a Meta-analysis of Host Responses to Infection A Thesis Submitted to the Faculty of Drexel University by Francis X. Bell in partial fulfillment of the requirements for the degree of Doctor of Philosophy November 2015 c Copyright 2015 Francis X. Bell. All Rights Reserved. ii Acknowledgments I would like to acknowledge and thank my advisor, Dr. Ahmet Sacan. Without his advice, support, and patience I would not have been able to accomplish all that I have. I would also like to thank my committee members and the Biomed Faculty that have guided me. I would like to give a special thanks for the members of the bioinformatics lab, in particular the members of the Sacan lab: Rehman Qureshi, Daisy Heng Yang, April Chunyu Zhao, and Yiqian Zhou. Thank you for creating a pleasant and friendly environment in the lab. I give the members of my family my sincerest gratitude for all that they have done for me. I cannot begin to repay my parents for their sacrifices. I am eternally grateful for everything they have done. The support of my sisters and their encouragement gave me the strength to persevere to the end. iii Table of Contents LIST OF TABLES.......................................................................... vii LIST OF FIGURES ........................................................................ xiv ABSTRACT ................................................................................ xvii 1. A BRIEF INTRODUCTION TO GENE EXPRESSION............................. 1 1.1 Central Dogma of Molecular Biology........................................... 1 1.1.1 Basic Transfers .......................................................... 1 1.1.2 Uncommon Transfers ................................................... 3 1.2 Gene Expression ................................................................. 4 1.2.1 Estimating Gene Expression ............................................ 4 1.2.2 DNA Microarrays ...................................................... -
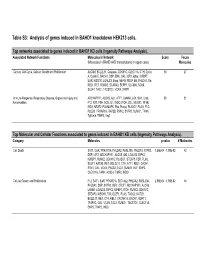
Table S3: Analysis of Genes Induced in BAHD1 Knockdown HEK213 Cells
Table S3: Analysis of genes induced in BAHD1 knockdown HEK213 cells. Top networks associated to genes induced in BAHD1 KD cells (Ingenuity Pathways Analysis). Associated Network Functions Molecules in Network Score Focus (Molecules in BAHD1-KD transcriptome in upper case) Molecules Cancer, Cell Cycle, Cellular Growth and Proliferation ALCAM, BCL2L11, Caspase, CDKN1C, CLEC11A, CTH, Cyclin 54 27 A, Cyclin E, DACH1, DSP, ERK, GAL, IGF2, Igfbp, IGFBP7, IL6R, KISS1R, LGALS3, Mmp, NEFM, PDGF BB, PHLDA1, Rb, RBL1, RET, RUNX2, SCARA3, SEPP1, SLC6A6, SOX8, SULF1, TAC1, TACSTD1, VCAN, WWP1 Immune Response, Respiratory Disease, Organismal Injury and ADCYAP1R1, ALOX5, Ap1, ATF1, CaMKII, COL13A1, Creb, 39 21 Abnormalities F12, F2R, FSH, hCG, IL1, INDO, ITCH, LDL, MEOX1, NFkB, NID1, NR2F2, P38 MAPK, Pka, Pkc(s), PLA2G7, PLAU, PLC, PLCD3, PRKAR1A, RAP2B, RIPK2, S1PR3, SUMO1, TANK, Tgf beta, TIMP2, Vegf Top Molecular and Cellular Functions associated to genes induced in BAHD1 KD cells (Ingenuity Pathways Analysis). Category Molecules p-value # Molecules Cell Death SAT1, IL6R, PRKAR1A, PHLDA2, RASL10A, PHLDA1, S1PR3, 1,56E-04 - 1,90E-02 43 DSP, IGF2, ADCYAP1R1, ALOX5, QKI, LGALS3, RIPK2, IGFBP7, RUNX2, CDKN1C, PLA2G7, STEAP3, F2R, PLAU, SULF1, KAT2B, RET, BCL2L11, CTH, ATF1, RBL1, DACH1, RTN1, GAL, VCAN, PACS2, TAC1, SUMO1, HLF, EMP2, CLEC11A, TANK, ACSL4, TIMP2, INDO Cellular Growth and Proliferation F12, SAT1, IL6R, PRKAR1A, SEC14L2, PHLDA2, RASL10A, 2,35E-04 - 1,90E-02 46 PHLDA1, DSP, S1PR3, IGF2, CRLF1, ADCYAP1R1, ALOX5, LAMB1, LGALS3, RIPK2, IGFBP7, -

A Network Inference Approach to Understanding Musculoskeletal
A NETWORK INFERENCE APPROACH TO UNDERSTANDING MUSCULOSKELETAL DISORDERS by NIL TURAN A thesis submitted to The University of Birmingham for the degree of Doctor of Philosophy College of Life and Environmental Sciences School of Biosciences The University of Birmingham June 2013 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Musculoskeletal disorders are among the most important health problem affecting the quality of life and contributing to a high burden on healthcare systems worldwide. Understanding the molecular mechanisms underlying these disorders is crucial for the development of efficient treatments. In this thesis, musculoskeletal disorders including muscle wasting, bone loss and cartilage deformation have been studied using systems biology approaches. Muscle wasting occurring as a systemic effect in COPD patients has been investigated with an integrative network inference approach. This work has lead to a model describing the relationship between muscle molecular and physiological response to training and systemic inflammatory mediators. This model has shown for the first time that oxygen dependent changes in the expression of epigenetic modifiers and not chronic inflammation may be causally linked to muscle dysfunction. -

BGGN 213 Pathway Analysis and the Interpretation of Gene Lists
BGGN 213 Pathway Analysis and the Interpretation of Gene Lists Barry Grant http://thegrantlab.org/bggn213 My high-throughput experiment generated a long list of genes/proteins… What do I do now? Pathway analysis! (a.k.a. geneset enrichment) Use bioinformatics methods to help extract biological meaning from such lists… Pathway analysis (a.k.a. geneset enrichment) Principle DEGs Pathway Differentially Pathway Expressed (geneset) Genes (DEGs) Enriched Not enriched • Variations of the math: overlap, ranking, networks... ➢ Not critical, different algorithms show similar performances • DEGs come from your experiment ➢ Critical, needs to be as clean as possible • Pathway genes (“geneset”) come from annotations ➢ Important, but typically not a competitive advantage Pathway analysis (a.k.a. geneset enrichment) Limitations • Post-transcriptional regulation is neglected • Directionality is hard to capture sensibly • e.g. IκBα/NF-κB • Tissue-specific variations of pathways are not annotated • e.g. NF-κB regulates metabolism, not inflammation, in adipocytes • Size bias: stats are influenced by the size of the pathway • Geneset annotation bias: can only discover what is already known • Non-model organisms: no high-quality genesets available • Many pathways/receptors converge to few regulators • e.g. tens of innate immune receptors activate 4 TFs: NF-kB, AP-1, IRF3/7, NFAT Starting point for pathway analysis: Your gene list • You have a list of genes/proteins of interest • You have quantitative data for each gene/protein • Fold change 228018_at F26A1.8NP_000192 -
A Mechanogenetic Model of Exercise-Induced Pulmonary Haemorrhage in the Thoroughbred Horse
G C A T T A C G G C A T genes Article A Mechanogenetic Model of Exercise-Induced Pulmonary Haemorrhage in the Thoroughbred Horse Sarah Blott 1,* , Hannah Cunningham 1, Laurène Malkowski 1 , Alexandra Brown 2 and Cyril Rauch 1,* 1 School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington LE12 5RD, UK; [email protected] (H.C.); [email protected] (L.M.) 2 Animal Health Trust, Lanwades Park, Kentford, Newmarket, Suffolk CB8 7UU, UK; [email protected] * Correspondence: [email protected] (S.B.); [email protected] (C.R.) Received: 30 September 2019; Accepted: 30 October 2019; Published: 1 November 2019 Abstract: Exercise-induced pulmonary haemorrhage (EIPH) occurs in horses performing high-intensity athletic activity. The application of physics principles to derive a ‘physical model’, which is coherent with existing physiology and cell biology data, shows that critical parameters for capillary rupture are cell–cell adhesion and cell stiffness (cytoskeleton organisation). Specifically, length of fracture in the capillary is a ratio between the energy involved in cell–cell adhesion and the stiffness of cells suggesting that if the adhesion diminishes and/or that the stiffness of cells increases EIPH is more likely to occur. To identify genes associated with relevant cellular or physiological phenotypes, the physical model was used in a post-genome-wide association study (GWAS) to define gene sets associated with the model parameters. The primary study was a GWAS of EIPH where the phenotype was based on weekly tracheal wash samples collected over a two-year period from 72 horses in a flat race training yard. -

BGGN 213 Pathway Analysis and the Interpretation of Gene Lists Lecture 15
BGGN 213 Pathway Analysis and the Interpretation of Gene Lists Lecture 15 Barry Grant http://thegrantlab.org/bggn213 Inputs Steps Reads R1 Reads R2 [optional] Control FastQ FastQ 1. Quality Reads R1 Reads R2 Control [optional] FastQC FastQ FastQ Treatment 2. Reference Alignment Genome (Mapping) UCSC Fasta TopHat2 Count Last day’s step Table 4. 3. data.frame Annotation Read Differential Counting expression UCSC GTF Count analysis! CuffLinks Table DESeq2 data.frame Volcano Plot Fold change vs P-value Significant (P < 0.01 & log2 > 2) My high-throughput experiment generated a long list of genes/proteins… What do I do now? Pathway analysis! (a.k.a. geneset enrichment) Use bioinformatics methods to help extract biological meaning from such lists… Inputs Steps Reads R1 Reads R2 [optional] Control FastQ FastQ 1. Quality Reads R1 Reads R2 Control [optional] FastQC 5. Gene set FastQ FastQ enrichment Treatment analysis 2. nalysis! Reference KEGG, GO, … Alignment Genome (Mapping) UCSC Fasta TopHat2 Count Table 4. 3. data.frame Annotation Read Differential Counting expression UCSC GTF Count analysis! CuffLinks Table DESeq2 data.frame Basic idea Differentially Expressed Genes (DEGs) Gene-sets (Pathways, annotations, etc...) Annotate... Basic idea Differentially Expressed Genes (DEGs) Gene-sets (Pathways, annotations, etc...) Annotate... Differentially Pathways Expressed Overlap... Genes (DEGs) Pathway analysis (geneset enrichment) Pathway analysis (a.k.a. geneset enrichment) Principle DEGs Pathway Differentially Pathway Expressed (geneset) Genes (DEGs) Enriched Not enriched • DEGs come from your experiment ➢ Critical, needs to be as clean as possible • Pathway genes (“geneset”) come from annotations ➢ Important, but typically not a competitive advantage • Variations of the math: overlap, ranking, networks..