Heat of Formation of Tetrafluorohydrazine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Argonne Report.Pdf
CONTENTS NOTATION ........................................................................................................................... xi ABSTRACT ........................................................................................................................... 1 1 INTRODUCTION ........................................................................................................... 5 1.1 Overview of the Emergency Response Guidebook ................................................ 5 1.2 Organization of this Report ..................................................................................... 7 2 GENERAL METHODOLOGY ....................................................................................... 9 2.1 TIH List ................................................................................................................... 10 2.1.1 Background ................................................................................................. 10 2.1.2 Changes in the TIH List for the ERG2012 ................................................. 11 2.2 Shipment and Release Scenarios ............................................................................ 11 2.2.1 Shipment Profiles ........................................................................................ 12 2.2.2 Treatment of Chemical Agents ................................................................... 14 2.3 Generics, Mixtures, and Solutions .......................................................................... 17 2.4 Analysis of Water-Reactive -

Particularly Hazardous Substances
Particularly Hazardous Substances In its Laboratory Standard, OSHA requires the establishment of additional protections for persons working with "Particularly Hazardous Substances" (PHS). OSHA defines these materials as "select" carcinogens, reproductive toxins and acutely toxic materials. Should you wish to add: explosive, violently reactive, pyrophoric and water-reactve materials to this category, the information is included. Carbon nanotubes have also been added due to their suspected carcinogenic properties. This table is designed to assist the laboratory in the identification of PHS, although it is not definitively conclusive or entirely comprehensive. *Notes on the proper use of this table appear on page 12. 1 6 5 2 3 4 Substance CAS National Toxicity National Program Carcinogen Toxin Acute Regulated OSHA Carcinogen Group IARC Carcinogen Toxin Reproductive Violently Reactive/ Explosive/Peroxide Forming/Pyrophoric A-a-C(2-Amino-9H-pyrido[2,3,b]indole) 2648-68-5 2B Acetal 105-57-7 yes Acetaldehyde 75-07-0 NTP AT 2B Acrolein (2-Propenal) 107-02-8 AT Acetamide 126850-14-4 2B 2-Acetylaminofluorene 53-96-3 NTP ORC Acrylamide 79-06-6 NTP 2B Acrylyl Chloride 814-68-6 AT Acrylonitrile 107-13-1 NTP ORC 2B Adriamycin 23214-92-8 NTP 2A Aflatoxins 1402-68-2 NTP 1 Allylamine 107-11-9 AT Alkylaluminums varies AT Allyl Chloride 107-05-1 AT ortho-Aminoazotoluene 97-56-3 NTP 2B para-aminoazobenzene 60-09-3 2B 4-Aminobiphenyl 92-67-1 NTP ORC 1 1-Amino-2-Methylanthraquinone 82-28-0 NTP (2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole) 67730-11-4 2B -

Ghs Reference Materials Health Hazard Criteria
APPENDIX F: GHS REFERENCE MATERIALS This appendix provides both an overview of GHS highly toxic hazard classification. A listing of Particularly Hazardous Substances that Carnegie Mellon University has published with their Chemical Hygiene plan is also provided as general reference. The list is useful cross check with GHS listings to determine which materials require prior approval for use BUT NO LIST IS COMPLETE you must check the SDS for possible additional chemicals rated as highly toxic. This appendix also provides the GHS (global harmonization system) for chemical hazard classification under the Hazard Communication Standard for highly toxic materials. This section provides overall information about categories under the classification of acute toxicity, mutagens’, reproductive and carcinogen hazards. When chemicals are rated on the GHS – Safety Data Sheet (SDS) as the following hazards then the PRIOR APPROVAL PROCESS WITH CHEMICAL HYGIENE OFFICER/COMMITTEE must be used: Acute toxicity category 1 and 2, Germ cell mutagenicity as a category 1A Substances known to induce heritable mutations in germ cells of humans and Category 1B: Substances which should be regarded as if they induce heritable mutations in the germ cells of humans, Reproductive Hazard as a category 1: Known or presumed human reproductive toxicants and Category 2; suspected human reproductive toxicant. Carcinogen as a Category 1 (includes 1A and 1B): Known or presumed human carcinogens, Category 2: Suspected human carcinogens. The Campus Chemical Hygiene Committee (Officer) must conduct a prior approval process. Appendix C Chemical Prior Approval Form on procedure for conducing prior approval. The following is from OSHA standard on the chemicals classifications that PCC Laboratory instructional operations shall use for defining the prior approval hazards. -

United States Patent Office Patented Mar
3,171,249 United States Patent Office Patented Mar. 2, 1965 i 2 fuel rocket engine. The above and other objects of this 3,171,249 PROPELLANT AND Rick |PROPULSSON METH invention will become apparent from the discussion OXD EMPLOYANG EYERAZINE WITH AMSNO which follows. TETRAZOLES The objects of this invention are accomplished by the Ronald E. Be, Canoga Park, Calif., assigner to use of compounds having the general formula: North American Aviatiosa, Bac. No Drawing. Fied Nov. 29, 1961, Ser. No. 155,803 NH2. (R) 8 Clains. (C. 60-35.4) N This invention relates to a novel rocket propellant. YS More particularly, this invention relates to a novel in 10 N--H proved rocket propellant and a method of operating a wherein x varies from 0 to 1 and R is selected from the rocket engine. class consisting of HCl, H2O, HNO3, and HCIO, as addi The criterion by which rocket propellants are classi tives to a hydrazine-based rocket fuel in an amount suffi fied is specific impulse, Is, defined as thrust in pounds 5 cient to depress the freezing point at least 40° C. while divided by the total mass flow of fuel and oxidizer in retaining about the same density impulse and specific pounds per second. Specific impulse is thus given in impulse. Hence, an embodiment of this invention com units of "seconds.” Oxidizer-fuel propulsion system prises a method of operating a rocket engine comprising compositions with a relatively high specific impulse are ejecting from the reaction chamber of the engine a gaseous known in the art. -

How to Identify and Report Hazardous Substances on The
OREGON COMMUNITY RIGHT TO KNOW AND PROTECTION ACT How to Identify and Report Hazardous Substances on the Hazardous Substance Information Survey February 2012 For assistance call the Mailing Address: Hazardous Substance Office of State Fire Marshal Information Hotline Community Right to Know Unit 4760 Portland Rd NE (503) 378-6835 Salem, OR 97305-1760 Toll Free (800) 454-6125 TDD (503) 390-4661 Monday – Friday Website: 8AM – 12PM and 1PM – 5PM http://www.oregon.gov/OSP/SFM/CR2K_Home.shtml Visit our website for more information: http://www.oregon.gov/OSP/SFM/CR2K_Home.shtml. These documents are currently available from our website: Blank Section D Chemical Form Blank Section E Additional Storage Location Form Survey Request Form Gas Conversion Chart Survey Mailing Schedule TABLE OF CONTENTS Introduction…………………………………………………………….. 1 Quick Steps to Complete the Survey …………………………………. 1 Reporting Requirements ……………………………...................... 2 What is a Hazardous Substance? ……………………………………... 3 What is a Reportable Quantity? ……………………………………….3 Reporting Compressed Gases ……………………………………….. 4 Liquefied and Cryogenic Gases …………………………………........ 4 Reporting Lead Acid Batteries ……………………………………….. 5 Tables for Completing the Survey ……………………………………... 6 Instructions and Definitions ……………………………………………. 7 Reporting Storage Locations …………………………………………..11 Frequently Asked Questions ………………………………………….. 13 EHS, 112r, PSM Questions …………………………………………… 14 EHS List ..............................................................................15 112(r) List ............................................................................18 -

FIREDOC Vocabulary List, 3Rd Edition
FIREDOC Vocabulary List, 3rd Edition II^STT United states Department of Commerce XI I National Institute of Standards and Technology NATIONAL INSTITUTE OF STANDARDS & TECHNOLOGY Research Information Center Gaithersburg, MD 20899 DATE DUE Demco, inc. 38-293 NIST Special Publication 779 FIREDOC Vocabulary List, 3rd Edition Nora H. Jason Center for Fire Research National Engineering Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NBSIR 85-3231 and NBSIR 87-3545) February 1990 U.S. Department of Commerce Robert A. Mosbacher, Secretary National Institute of Standards and Technology John W. Lyons, Director National Institute of Standards U.S. Government Printing Office For sale by the Superintendent and Technology Washington: 1990 of Documents Special Publication 779 U.S. Government Printing Office (Supersedes NBSIR 85-3231 Washington, DC 20402 and NBSIR 87-3545) Natl. Inst. Stand. Technol. Spec. Publ. 779 104 pages (Feb. 1990) CODEN: NSPUE2 Contents Acknowledgments v Introduction 1 Symbol Notation 1 Terms Beginning with A 3 Terms Beginning with B 11 Terms Beginning with C 15 Terms Beginning with D 23 Terms Beginning with E 29 Terms Beginning with F 33 Terms Beginning with G 43 Terms Beginning with H 45 Terms Beginning with I 51 Terms Beginning with J 55 Terms Beginning with K 57 Terms Beginning with L 59 Terms Beginning with M 63 Terms Beginning with N 69 Terms Beginning with O 71 Terms Beginning with P 73 Terms Beginning with Q 81 Terms Beginning with R 83 Terms Beginning with S 87 iii Terms Beginning with T 95 Terms Beginning with U 101 Terms Beginning with V 103 Terms Beginning with W 105 Terms Beginning with X 107 Terms Beginning with Y 109 Terms Beginning with Z Ill iv Acknowledgments Richard D. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -
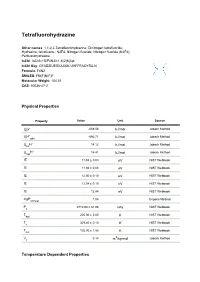
Tetrafluorohydrazine Datasheet
Tetrafluorohydrazine Other names: 1,1,2,2-Tetrafluorohydrazine; Dinitrogen tetrafluoride; Hydrazine, tetrafluoro-; N2F4; Nitrogen fluoride; Nitrogen fluoride (N2F4); Perfluorohydrazine. InChI: InChI=1S/F4N2/c1-5(2)6(3)4 InChI Key: GFADZIUESKAXAK-UHFFFAOYSA-N Formula: F4N2 SMILES: FN(F)N(F)F Molecular Weight: 104.01 CAS: 10036-47-2 Physical Properties Property Value Unit Source ∆ G° -608.56 kJ/mol Joback Method f ∆ H° -692.71 Joback Method f gas kJ/mol ∆ H° 14.12 Joback Method fus kJ/mol ∆ H° 16.41 Joback Method vap kJ/mol IE 11.94 ± 0.03 eV NIST Webbook IE 11.94 ± 0.03 eV NIST Webbook IE 12.00 ± 0.10 eV NIST Webbook IE 12.04 ± 0.10 eV NIST Webbook IE 12.84 eV NIST Webbook logP 1.04 Crippen Method oct/wat P 3710.00 ± 81.06 NIST Webbook c kPa T 200.00 ± 2.00 NIST Webbook boil K T 309.40 ± 0.10 NIST Webbook c K T 105.00 ± 1.50 NIST Webbook fus K V 0.14 3 Joback Method c m /kg-mol Temperature Dependent Properties Property Value Unit Temperature (K) Source C 59.86 J/mol×K 221.36 Joback Method p,gas ∆ H 26.40 200.0 NIST Webbook vap kJ/mol Sources Joback Method: https://en.wikipedia.org/wiki/Joback_method NIST Webbook: http://webbook.nist.gov/cgi/inchi/InChI=1S/F4N2/c1-5(2)6(3)4 Crippen Method: http://pubs.acs.org/doi/abs/10.1021/ci990307l Legend C : Ideal gas heat capacity (J/mol×K). p,gas ∆ G°: Standard Gibbs free energy of formation (kJ/mol). -

Table 4: Protective Action Criteria (PAC) Rev. 29 Based on Applicable
Table 4: Protective Action Criteria (PAC) Rev. 29a based on applicable 60-minute AEGLs, ERPGs, or TEELs. The chemicals are 3 listed in alphabetical order and the values are presented in mg/m . June 2018 Table 4 is an alphabetical list of the chemical substances and their corresponding PAC values in mass per unit volume (mg/m3). The conversion of ppm to mg/m3 was carried out assuming normal temperature and pressure, 25°C and 760 mm Hg. The columns presented in Table 4 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this alphabetical listing Chemical Name The name of the chemical substance submitted to the PAC development team CASRN The Chemical Abstracts Service Registry Number1 for this chemical PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value Chemicals for which AEGLs are available have their chemical name, CASRN, and AEGL values displayed in a bolded and larger font. Chemicals for which ERPGs are available, but not AEGLs, have their chemical name, CASRN, and ERPG values displayed in a bolded font. Chemicals for which TEELs are available, but no AEGLs or ERPGs, have their chemical name, CASRN, and values displayed using a regular font. Additional information on PAC values and TEEL values and links to other sources of information is provided on the Subcommittee on Consequence Assessment and Protective Actions (SCAPA) webpage at http://orise.orau.gov/emi/scapa/default.htm. -

LIQUID Rocker PROPELLANT COMPATIBILITY TESTING By
1 , 7 I I LIQUID ROCKEr PROPELLANT COMPATIBILITY TESTING by William T. McFarlen Rocketdyne A Division of North American Aviation, Inc., Canoga Park, California SATURN HISTORY DOCUM�NT University of Ale bema Peeee�ch Institute History of Scie,ice & Technology Group Dlite----------D oc.No. _______ _ Presented at the American Society for Testing and Materials Technical Division O Symposium. on Aerospace Test Methods and Materials Boston, Massachuseits 26-28 June 1967 LIQUID ROCKET PROPELLANT COMPATIBILITY TESTING by William T. McFarlen Materials and Processes Rocketdyne A Division of North American Aviation, Inc., Canoga Park, California ABSTRACT Material-propellant compatibility as related to liquid rocket propulsion system design criteria is discussed and applicable test methods to derive usable design data are presented. Test methods, with emphasis on metal lic materials, are discussed and the shortcomings of a number of these test methods are pointed out. These tests include static immersion tests, stress-corrosion tests, flow tests, impact tests, and tests to determine the effect of cracks and notches in metals on compatibility. A general outline for the evaluation of metallic and nonmetallic materials with respect to propellant compatibility is presented. 1 INTRODUCTION One of the prime considerations in the design of a liquid rocket propul sion system is the possible interaction between the propellants and the materials of construction. Examples of such interactions are simple chem ical dissolution, solubility effects, selective chemical attack, stress corrosion, fatigue corrosion, thermal ignition, effect of the environment on notched strength, and deterioration of other mechanical properties. Materials evaluation in this area falls into the category of material compatibility or material-propellant compatibility. -

Supplementary Information Cocrystal Design by Network-Based Link Prediction
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is © The Royal Society of Chemistry 2019 Supplementary Information Cocrystal design by network-based link prediction Jan-Joris Devogelaer, Sander J.T. Brugman, Hugo Meekes, Paul Tinnemans, Elias Vlieg, René de Gelder Table of Contents S1 Materials and syntheses page 1 S2 Single-crystal X-ray structure determinations, ORTEP plots and H-Bonding page 6 a) CCDC 1940959 = p1812d: 4,4’-bipyridine + resorcinol b) CCDC 1940950 = p1823c: 1,2-di(4-pyridyl)ethylene + 4-aminobenzoic acid c) CCDC 1940956 = p1823a: 1,2-di(4-pyridyl)ethylene + 4-aminobenzoic acid dihydrate d) CCDC 1940954 = p1907a: 1,2-di(4-pyridyl)ethylene + sebacic acid e) CCDC 1940949 = p1818a: 4,4’-bipyridine + suberic acid f) CCDC 1940953 = p1922b: 1,2-di(4-pyridyl)ethylene + oxalic acid g) CCDC 1940955 = p1914b: 1,2-di(4-pyridyl)ethylene + oxalic acid dihydrate i) CCDC 1940951 = p1908a: 1,2-bis(4-pyridyl)ethane + salicylic acid j) CCDC 1940958 = p1926b: 1,2-di(4-pyridyl)ethylene + 4-nitrobenzoic acid k) CCDC 1940952 = p1910a: 1,2-di(4-pyridyl)ethylene + phthalic acid l) CCDC 1940957 = p1913a: 1,2-di(4-pyridyl)ethylene + malonic acid S3 Top 100 predictions of the scoring method page 17 S4 Predefined lists of common solvents and gases page 21 S1 - Materials and syntheses We first present the materials in Table S1, after which we discuss the protocol used to obtain crystals suitable for single-crystal X-ray diffraction. Besides the two-component structures shown in Table 2, an additional cocrystal dihydrate (c) and salt dihydrate (g) were found. -

New York City Department of Environmental Protection Community Right-To-Know: List of Hazardous Substances
New York City Department of Environmental Protection Community Right-to-Know: List of Hazardous Substances Updated: 12/2015 Definitions SARA = The federal Superfund Amendments and Reauthorization Act (enacted in 1986). Title III of SARA, known as the Emergency Planning and Community Right-to-Act, sets requirements for hazardous chemicals, improves the public’s access to information on chemical hazards in their community, and establishes reporting responsibilities for facilities that store, use, and/or release hazardous chemicals. RQ = Reportable Quantity. An amount entered in this column indicates the substance may be reportable under §304 of SARA Title III. Amount is in pounds, a "K" represents 1,000 pounds. An asterisk following the Reporting Quantity (i.e. 5000*) will indicate that reporting of releases is not required if the diameter of the pieces of the solid metal released is equal to or exceeds 100 micrometers (0.004 inches). TPQ = Threshold Planning Quantity. An amount entered in this column reads in pounds and indicates the substance is an Extremely Hazardous Substance (EHS), and may require reporting under sections 302, 304 & 312 of SARA Title III. A TPQ with a slash (/) indicates a "split" TPQ. The number to the left of the slash is the substance's TPQ only if the substance is present in the form of a fine powder (particle size less than 100 microns), molten or in solution, or reacts with water (NFPA rating = 2, 3 or 4). The TPQ is 10,000 lb if the substance is present in other forms. A star (*) in the 313 column= The substance is reportable under §313 of SARA Title III.