Particularly Hazardous Substances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flavour Management by Citric Acid Negative MLF Starter Cultures Authors: Carsten Heinemeyer, 2B Fermcontrol; Ulrich Hamm DLR-RNH Bad Kreuznach, Germany; Dr
Flavour management by citric acid negative MLF starter cultures Authors: Carsten Heinemeyer, 2B FermControl; Ulrich Hamm DLR-RNH Bad Kreuznach, Germany; Dr. Jürgen Fröhlich, University of Mainz General will be completed in all fermentations. Additionally, acetic acid and a certain residual profile of the metabolic intermediates The malo-lactic fermentation (MLF) is a commonly used remain in the wine. method to convert the aggressive malic acid to lactic acid. This conversion results in a reduction of the titratable acidity, which New MLF Starter Cultures is desired mainly in red wine but also in numerous white wines. The development of a citric acid negative MLF starter culture This process will be done either by the indigenous flora of LAB gave a new opportunity to avoid diacetyl and acetic acid or by selected strains of LAB e.g. Oenococcus oeni. During the production from the citric acid degradation. The fermentation MLF Oenococcus oeni does not convert only malic acid into lactic with a citric acid negative MLF starter culture will best preserve acid, numerous amounts of aroma active by-products will also be the varietal character of the wine. produced. The best known is diacetyl, which gives buttery notes to the wine. Diacetyl will be produced during the MLF by the In a comprehensive four year study at the wine research institute conversion of the natural citric acid in the wine by Oenococcus DLR-RNH Bad Kreuznach in Rhineland-Palatinate-Germany, oeni (Jan Clair Nielsen 1999). Apart from the diacetyl formation different MLF starter strains were tested under practical by Oenococcus oeni, numerous intermediate by-products are winemaking conditions. -

(A) 1) at First a Pair of Each 10G. of Sample Was Extracted with Ether by the Soxhlet's Apparatus for a Week
52 [vol. 6, THE DISTRIBUTION OF PHOSPHORUS IN COW'S MILK AND THE SCHEME FOR THE SEPARATION OF PHOSPHATIDES By Rinjiro SASAKI (From the Institute of Agricultural Chemistry, College of Agriculture, Tokyo Imperial University) (Received 9th September 1930) A. The Distribution of Phosphorus in Cow's milk In order to extract the phosphatides, it is necessary first to determine the distribution of phosphorus in cow's milk. Liquid milk can not be extracted with ether or with other organic solvents owing to its large content of water. The substance applied in this experiment is the milk powder, dried by the Buflovak drum drier below 70•Ž. Its content of moisture is 4.498%, deter mined by the method of drying in the air bath of 105•Ž. The content of total phosphorus was determined(5) in the ash of sample which was burned with a little of fusing mixture. In 100g. of milk powder In 100g. of dry matter Total phosphorus 0.745g. 0.781g. The amounts of phosphorus, which are soluble in the various organic sol vents, were determined by extracting with three solvents in the following orders. (A) Ether-Acetone-Alcohol (B) Acetone-Ether-Alcohol (C) Alcohol-Ether-Acetone Experiment (A) 1) At first a pair of each 10g. of sample was extracted with ether by the Soxhlet's apparatus for a week. After the extraction had been comp leted, the extract was freed from ether and weighed. In 100g.of milkpowder In 100g.of dry matter Total ether matters soluble 3.609g. 3.779g. 2) The above extract was dissolved in a very little of ether. -
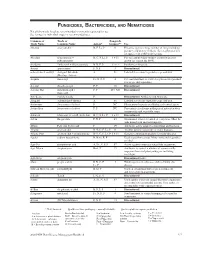
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

Phosphorus Oxychloride CAS No
Product Safety Summary Phosphorus Oxychloride CAS No. 10025-87-3 This Product Safety Summary is intended to provide a general overview of the chemical substance. The information in the summary is basic information and is not intended to provide emergency response information, medical information or treatment information. The summary should not be used to provide in-depth safety and health information. In-depth safety and health information can be found in the Safety Data Sheet (SDS) for the chemical substance. Names Phosphorus oxychloride Phosphorus trichloride oxide Phosphoric trichloride POCl 3 Product Overview Solvay Novecare does not sell phosphorus oxychloride directly to consumers. Phosphorus oxychloride (POCl3) is used as a chemical intermediate to produce a variety of products which are used in several applications including manufacture of triarylphosphate esters which are used as flame retardants as well as an intermediate in the production of pharmaceutical, textile and agricultural chemicals. Phosphorus oxychloride is used in industrial applications and other processes where workplace exposures can occur. Consumer exposure does not occur as phosphorus oxychloride is not used in any commercially available product. Phosphorus oxychloride is dangerous to human health. Phosphorus oxychloride may be fatal if inhaled, highly toxic if swallowed, harmful if absorbed through skin and can cause severe burns which may result in scarring. Phosphorus oxychloride is consumed in manufacturing processes. POCl3 can make its way into the environment through unintentional releases (spills). POCl3 will not bioaccumulate but is not biodegradable. POCl3 in higher concentrations can be harmful to aquatic life due to formation of acids from the hydrolysis of POCl3. When released into the atmosphere, phosphorus oxychloride exists as vapor. -

The Use and Storage of Methyl Isocyanate (MIC) at Bayer Cropscience
Summary The Use and Storage of Methyl Isocyanate (MIC) at Bayer CropScience The use of hazardous chemicals such as methyl isocyanate can be a significant concern to the residents of communities adjacent to chemical facilities, but is often an integral, necessary part of the chemical manufacturing process. In order to ensure that chemical manufacturing takes place in a manner that is safe for workers, members of the local community, and the environment, the philosophy of inherently safer processing can be used to identify opportuni ties to eliminate or reduce the hazards associated with chemical processing. However, the concepts of inherently safer process analysis have not yet been adopted in all chemical manu facturing plants. This report presents a possible framework to help plant managers choose between alternative processing options—considering factors such as environmental impact and product yield as well as safety—to develop a chemical manufacturing system. n 2008, an explosion at the Bayer CropScience chemical production Iplant in Institute, West Virginia, resulted in the deaths of two employees, a fire within the production unit, and extensive damage to nearby structures. The accident drew renewed attention to the fact that the Bayer facility manufac tured and stored methyl isocyanate, or MIC—a volatile, highly toxic chemical used in the production of carbamate pesticides and the agent responsible for thousands of deaths in Bhopal, India, in 1984. In the Institute incident, debris Figure 1. The Bayer CropScience facility at Insitute, WV. from the blast hit the shield surrounding Google Earth satellite image: © 2012 Google a MIC storage tank, and although the container was not damaged, an investiga tion by the U.S. -

Methanesulfonyl Chloride
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

5 Phosphorus Oxychloride1 Acute Exposure Guideline Levels
Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 10 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 10 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This project was supported by Contract No. W81K04-06-D-0023 and EP-W-09-007 be- tween the National Academy of Sciences and the U.S. Department of Defense and the U.S. Environmental Protection Agency. Any opinions, findings, conclusions, or recom- mendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the organizations or agencies that provided support for this project. International Standard Book Number-13: 978-0-309-21987-7 International Standard Book Number-10: 0-309-21987-6 Additional copies of this report are available from The National Academies Press 500 Fifth Street, NW Box 285 Washington, DC 20055 800-624-6242 202-334-3313 (in the Washington metropolitan area) http://www.nap.edu Copyright 2011 by the National Academy of Sciences. -

The Decomposition Kinetics of Peracetic Acid and Hydrogen Peroxide in Municipal Wastewaters
Disinfection Forum No 10, October 2015 The Decomposition Kinetics of Peracetic Acid and Hydrogen Peroxide in Municipal Wastewaters INTRODUCTION Efficient control of microbial populations in municipal wastewater using peracetic acid (PAA) requires an understanding of the PAA decomposition kinetics. This knowledge is critical to ensure the proper dosing of PAA needed to achieve an adequate concentration within the contact time of the disinfection chamber. In addition, the impact of PAA on the environment, post-discharge into the receiving water body, also is dependent upon the longevity of the PAA in the environment, before decomposing to acetic acid, oxygen and water. As a result, the decomposition kinetics of PAA may have a significant impact on aquatic and environmental toxicity. PAA is not manufactured as a pure compound. The solution exists as an equilibrium mixture of PAA, hydrogen peroxide, acetic acid, and water: ↔ + + Acetic Acid Hydrogen Peroxide Peracetic Acid Water PeroxyChem’s VigorOx® WWT II Wastewater Disinfection Technology contains 15% peracetic acid by weight and 23% hydrogen peroxide as delivered. Although hydrogen peroxide is present in the formulation, peracetic acid is considered to be the active component for disinfection1 in wastewater. There have been several published studies investigating the decomposition kinetics of PAA in different water matrices, including municipal wastewater2-7. Yuan7 states that PAA may be consumed in the following three competitive reactions: 1. Spontaneous decomposition 2 CH3CO3H à 2 CH3CO2H + O2 Eq (1) 2. Hydrolysis CH3CO3H + H2O à CH3CO2H + H2O2 Eq (2) 3. Transition metal catalyzed decomposition + CH3CO3H + M à CH3CO2H + O2 + other products Eq (3) At neutral pH’s, both peracetic acid and hydrogen peroxide can be rapidly consumed by these reactions7 (hydrogen peroxide will decompose to water and oxygen via 2H2O2 à 2H2O + O2). -

United States Patent (19) 11 Patent Number: 4,496,778 Myers Et Al
United States Patent (19) 11 Patent Number: 4,496,778 Myers et al. (45) Date of Patent: Jan. 29, 1985 (54) PROCESS FOR THE HYDROXYLATION OF 56 References Cited OLEFINS USING MOLECULAR OXYGEN, U.S. PATENT DOCUMENTS ANOSMIUM CONTAINING CATALYST, A COPPER CO-CATALYST, AND AN 2,773, 101 12/1956 Smith et al. ......................... 568/860 AROMATIC AMINE BASED PROMOTER 3,317,592 5/1967 Maclean et al. ... 568/860 3,337,635 8/1967 Norton et al. ....... 568/860 75 Inventors: Richard S. Myers, Fairlawn; Robert 4,390,739 6/1983 Michaelson et al. .... ..., 568/860 C. Michaelson, Waldwick; Richard FOREIGN PATENT DOCUMENTS G. Austin, Ridgewood, all of N.J. 32522 8/1974 Japan ................................... 568/860 73) Assignee: Exxon Research & Engineering Co., Primary Examiner-J. E. Evans Florham Park, N.J. Attorney, Agent, or Firm-Robert A. Maggio 21 Appl. No.: 538,190 57 ABSTRACT A process directed to the hydroxylation of olefins by 22 Filed: Oct. 3, 1983 reacting said olefins in the presence of oxygen, water, and a catalyst composition comprising (i) a catalytically 51 Int. Cl. ...................... C07C 29/04; CO7C 31/18; active osmium containing compound, (ii) a Co-catalyst C07C 31/22; CO7C 31/42 I comprising a copper containing compound such as 52 U.S.C. ................................. 568/860; 260/.397.2; CuBr2, and (iii) a Co-catalyst II capable of increasing 560/186; 562/587; 568/811; 568/821; 568/833; the rate and/or selectivity of the hydroxylation reac 568/838; 568/847 tion, such as pyridine is disclosed. 58 Field of Search .............. -

Peracetic Acid Processing
Peracetic Acid Processing Identification Chemical Name(s): CAS Number: peroxyacetic acid, ethaneperoxic acid 79-21-0 Other Names: Other Codes: per acid, periacetic acid, PAA NIOSH Registry Number: SD8750000 TRI Chemical ID: 000079210 UN/ID Number: UN3105 Summary Recommendation Synthetic / Allowed or Suggested Non-Synthetic: Prohibited: Annotation: Synthetic Allowed (consensus) Allowed only for direct food contact for use in wash water. Allowed as a (consensus) sanitizer on surfaces in contact with organic food. (consensus) From hydrogen peroxide and fermented acetic acid sources only. (Not discussed by processing reviewers--see discussion of source under Crops PAA TAP review.) Characterization Composition: C2H4O3. Peracetic acid is a mixture of acetic acid (CH3COOH) and hydrogen peroxide (H2O2) in an aqueous solution. Acetic acid is the principle component of vinegar. Hydrogen peroxide has been previously recommended by the NOSB for the National List in processing (synthetic, allowed at Austin, 1995). Properties: It is a very strong oxidizing agent and has stronger oxidation potential than chlorine or chlorine dioxide. Liquid, clear, and colorless with no foaming capability. It has a strong pungent acetic acid odor, and the pH is acid (2.8). Specific gravity is 1.114 and weighs 9.28 pounds per gallon. Stable upon transport. How Made: Peracetic acid (PAA) is produced by reacting acetic acid and hydrogen peroxide. The reaction is allowed to continue for up to ten days in order to achieve high yields of product according to the following equation. O O || || CH3-C-OH + H2O2 CH3C-O-OH + H2O acetic acid hydrogen peroxyacetic peroxide acid Due to reaction limitations, PAA generation can be up to 15% with residual levels of hydrogen peroxide (up to 25%) and acetic acid (up to 35%) with water up to 25%. -

United States Patent Office Patented Apr
3,030,421 United States Patent Office Patented Apr. 17, 1962 2 3,030,421 perature and under a reduced pressure. In the case of PROCESS FOR PREPARNG TRHYDROXY most of the catalysts the reaction products, which are free METHYL-PHOSPHINE s from solvents, solidify already when being cooled to room Martin Reuter and Ludwig Ortane, Frankfurt an Main, temperature. If, in the case of some catalysts, they Germany, assignors to Farbwerke Hoechst Aktienger 5 solidify only at a lower temperature and still contain to sellschaft vormas Meister Lucius & Briining, Frank a greater extent oily by-products and/or phosphonium furt am Main, Germany, a corporation of Gerinally hydroxide, they can be separated from the latter by filter No Drawing. Fied Jaa. 14, 1958, Ser. No. 708,764 ing or pressing. - - - Claims priority, application Germany Jan. 23, 1957 It is to be assumed that the crystalline main product 6 Canas. (C. 260-606.5) O of the present invention constitutes the hitherto unknown We have found that a new and valuable phosphorus trihydroxymethyl-phosphine. Main and by-products are compound carrying hydroxymethyl groups at the phos easily soluble in water and methanol and sparingly solu phorus atom can be prepared by reacting 1 mol of formal ble in fat dissolvers. dehyde with 4 mol of phosphine, preferably in the pres The reaction products of the invention can be used as ence of water, and in the presence of Small quantities of 5 insecticides, additives for lubricants, flame-proofing agents finely distributed metals that do not belong to the alkali for wood and textiles and as intermediates for these sub metals or alkaline earth metals and/or their compounds Staces. -

Cellular Uptake and Toxicological Effects of Differently Sized Zinc Oxide Nanoparticles in Intestinal Cells †
toxics Article Cellular Uptake and Toxicological Effects of Differently Sized Zinc Oxide Nanoparticles in Intestinal Cells † Anna Mittag 1,* , Christian Hoera 2, Alexander Kämpfe 2 , Martin Westermann 3, Jochen Kuckelkorn 4, Thomas Schneider 1 and Michael Glei 1 1 Department of Nutritional Toxicology, Institute of Nutritional Sciences, Friedrich Schiller University Jena, Dornburger Straße 24, 07743 Jena, Germany; [email protected] (T.S.); [email protected] (M.G.) 2 German Environment Agency, Swimming Pool Water, Chemical Analytics, Heinrich-Heine-Straße 12, 08645 Bad Elster, Germany; [email protected] (C.H.); [email protected] (A.K.) 3 Electron Microscopy Centre, Friedrich Schiller University Jena, Ziegelmühlenweg 1, 07743 Jena, Germany; [email protected] 4 German Environment Agency, Toxicology of Drinking Water and Swimming Pool Water, Heinrich-Heine-Straße 12, 08645 Bad Elster, Germany; [email protected] * Correspondence: [email protected] † In respectful memory of Dr. Tamara Grummt. Abstract: Due to their beneficial properties, the use of zinc oxide nanoparticles (ZnO NP) is constantly increasing, especially in consumer-related areas, such as food packaging and food additives, which is leading to an increased oral uptake of ZnO NP. Consequently, the aim of our study was to investigate the cellular uptake of two differently sized ZnO NP (<50 nm and <100 nm; 12–1229 µmol/L) using two human intestinal cell lines (Caco-2 and LT97) and to examine the possible resulting toxic effects. ZnO NP (<50 nm and <100 nm) were internalized by both cell lines and led to intracellular changes. Citation: Mittag, A.; Hoera, C.; Kämpfe, A.; Westermann, M.; Both ZnO NP caused time- and dose-dependent cytotoxic effects, especially at concentrations of Kuckelkorn, J.; Schneider, T.; Glei, M.