Assessment of Portable HAZMAT Sensors for First Responders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On the Photoionization of Large Molecules
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector ________ ~~__ On the Photoionization of Large Molecules C. H. Becker and K. J. Wu* Molecular Phvxics L‘tbordtorv, SRI Irlternatmnal, Menlo Park. ialifornia, USA There is no apparent limit to the size of a molecule for which photoionization can occur. It is argued that it is difficult to obtain useful photoionization mass spectra of peptides (above - 2000 u), proteins, and oligonucleotides, because of the high internal energy of these polar molecules as a result of the desorption event and because vibrationally excited radical cations readily fragment. Evidence to support this hypothesis is presented from the 1%nm single-photon ionization 61’1) mass spectra of the cyclic decapeptide gramicidin S and of fullerencs, from null SPI results with the linear peptides substance P and gramicidin D and oligonucleotides, and from a variety of data found in the literature. The literature data include mass spectra from jet-cooled peptides, perfluorinated polyethers, collisional ioniza- tion of small neutral peptides, and the ultraviolet photoelectron spectroscopy of polymeric solids. (1 Am Sot Mass Spcctrom 1995, 6, 883-888) uch recent effort in mass spectrometry has (MALD), peptides and proteins show significant de- been directed toward the analysis of peptides grees of metastable decay that increases with the mass M and proteins [l, 21, DNA [3, 41, and other of the molecule [ll]. Even for the MALD technique, large biopolymers. Successful analytical approaches to considered currently to be the “gentlest” of all stimu- date have used the direct ionization associated with lated desorption techniques in that it permits observa- the desorption process, especially with laser desorp- tion of the quasimolecular ions of large proteins and tion. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
![Immediately Dangerous to Life Or Health (Idlh) Value Profile for Chlorine Pentafluoride [Cas No. 13637-63-3] and Bromine Pentafl](https://docslib.b-cdn.net/cover/6027/immediately-dangerous-to-life-or-health-idlh-value-profile-for-chlorine-pentafluoride-cas-no-13637-63-3-and-bromine-pentafl-116027.webp)
Immediately Dangerous to Life Or Health (Idlh) Value Profile for Chlorine Pentafluoride [Cas No. 13637-63-3] and Bromine Pentafl
External Review Draft March 2015 1 2 3 4 5 6 7 IMMEDIATELY DANGEROUS TO LIFE OR HEALTH (IDLH) VALUE PROFILE 8 9 10 11 FOR 12 13 14 15 CHLORINE PENTAFLUORIDE [CAS NO. 13637-63-3] 16 17 AND 18 19 BROMINE PENTAFLUORIDE [CAS NO. 7789-30-2] 20 21 22 23 24 25 26 Department of Health and Human Services 27 Centers for Disease Control and Prevention 28 National Institute for Occupational Safety and Health This information is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the National Institute for Occupational Safety and Health. It does not represent and should not be construed to represent any agency determination or policy. i External Review Draft March 2015 1 DISCLAIMER 2 Mention of any company or product does not constitute endorsement by the National Institute for Occupational 3 Safety and Health (NIOSH). In addition, citations of Web sites external to NIOSH do not constitute NIOSH 4 endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not 5 responsible for the content of these Web sites. 6 7 ORDERING INFORMATION 8 This document is in the public domain and may be freely copied or reprinted. To receive NIOSH documents or 9 other information about occupational safety and health topics, contact NIOSH at 10 Telephone: 1-800-CDC-INFO (1-800-232-4636) 11 TTY: 1-888-232-6348 12 E-mail: [email protected] 13 14 or visit the NIOSH Web site at www.cdc.gov/niosh. -

The Use and Storage of Methyl Isocyanate (MIC) at Bayer Cropscience
Summary The Use and Storage of Methyl Isocyanate (MIC) at Bayer CropScience The use of hazardous chemicals such as methyl isocyanate can be a significant concern to the residents of communities adjacent to chemical facilities, but is often an integral, necessary part of the chemical manufacturing process. In order to ensure that chemical manufacturing takes place in a manner that is safe for workers, members of the local community, and the environment, the philosophy of inherently safer processing can be used to identify opportuni ties to eliminate or reduce the hazards associated with chemical processing. However, the concepts of inherently safer process analysis have not yet been adopted in all chemical manu facturing plants. This report presents a possible framework to help plant managers choose between alternative processing options—considering factors such as environmental impact and product yield as well as safety—to develop a chemical manufacturing system. n 2008, an explosion at the Bayer CropScience chemical production Iplant in Institute, West Virginia, resulted in the deaths of two employees, a fire within the production unit, and extensive damage to nearby structures. The accident drew renewed attention to the fact that the Bayer facility manufac tured and stored methyl isocyanate, or MIC—a volatile, highly toxic chemical used in the production of carbamate pesticides and the agent responsible for thousands of deaths in Bhopal, India, in 1984. In the Institute incident, debris Figure 1. The Bayer CropScience facility at Insitute, WV. from the blast hit the shield surrounding Google Earth satellite image: © 2012 Google a MIC storage tank, and although the container was not damaged, an investiga tion by the U.S. -

Improved Treatment of the Photoionization Process in the Laser Induced Optical Breakdown in the Laser Tissue
U.P.B. Sci. Bull., Series A, Vol. 81, Iss. 4, 2019 ISSN 1223-7027 IMPROVED TREATMENT OF THE PHOTOIONIZATION PROCESS IN THE LASER INDUCED OPTICAL BREAKDOWN IN THE LASER TISSUE Violeta PETROVIĆ1 and Hristina DELIBAŠIĆ1 The development of laser technology led to the discovery that laser-living tissue (cells) interactions have significant biomedical applications and can be used to perform precise surgical procedures of 'water-like' tissues (such as the eye). When the focus is located within transparent biological cells and tissues, nonlinear absorption processes initiate a laser induced optical breakdown. The threshold for breakdown is defined by a certain critical free electron density. An in depth understanding of these processes orientated our theoretical research to the development of rate equations describing electron density growth in a transparent biological media exposed to a femtosecond laser pulse. In order to provide an accurate theoretical model and to predict damage occurrence, we took into account the losses through diffusion of electrons out of the focal volume, cascade ionization and the model of photoionization based on the standard Keldysh and ADK theory. Keywords: laser-induced breakdown, avalanche process, Keldysh theory. 1. Introduction The advent of high-power lasers opened the door for a wide range of laser application [1, 2, 3]. The effect known as the breakdown is a very important topic due to its role in laser applications. Breakdown is an effect which can be produced by high electric field strengths. This means that after a spark the medium becomes electrically conducting. Laser-induced breakdown (LIB) can occur in any media: solid, liquid, or gas. -
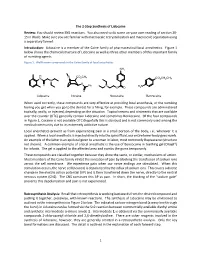
1 the 2-Step Synthesis of Lidocaine Review
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

Safety Data Sheet According to 29CFR1910/1200 and GHS Rev
Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. 3 Effective date : 01.06.2015 Page 1 of 7 Methylene Blue, Loeffler's SECTION 1 : Identification of the substance/mixture and of the supplier Product name : Methylene Blue, Loeffler's Manufacturer/Supplier Trade name: Manufacturer/Supplier Article number: S25432 Recommended uses of the product and uses restrictions on use: Manufacturer Details: AquaPhoenix Scientific 9 Barnhart Drive, Hanover, PA 17331 Supplier Details: Fisher Science Education 15 Jet View Drive, Rochester, NY 14624 Emergency telephone number: Fisher Science Education Emergency Telephone No.: 800-535-5053 SECTION 2 : Hazards identification Classification of the substance or mixture: Flammable Flammable solids, category 2 Flammable liq. 2 Signal word :Danger Hazard statements: Highly flammable liquid and vapour Precautionary statements: If medical advice is needed, have product container or label at hand Keep out of reach of children Read label before use Keep away from heat/sparks/open flames/hot surfaces. No smoking Keep container tightly closed Ground/bond container and receiving equipment Use explosion-proof electrical/ventilating/light/…/equipment Use only non-sparking tools Take precautionary measures against static discharge Wear protective gloves/protective clothing/eye protection/face protection IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower In case of fire: Use … for extinction Store in a well ventilated place. Keep cool Dispose of contents/container to … Other Non-GHS Classification: WHMIS Created by Global Safety Management, Inc. -Tel: 1-813-435-5161 - www.gsmsds.com Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Catalyzed Reaction of Isocyanates (RNCO) with Water
Catalyzed Reaction of Isocyanates (RNCO) with Water Mark E. Wolf,y Jonathon E. Vandezande,z,{ and Henry F. Schaefer III∗,y yCenter for Computational Quantum Chemistry, University of Georgia, 140 Cedar Street, Athens, Georgia 30602, USA z1st Source Research, 617 Hutton St., Raleigh, North Carolina 27606, USA {Zymergen, 5980 Horton Street, Suite 105, Emeryville, California 94608, USA E-mail: [email protected] Phone: +1 706 542-2067. Fax: +1 706 542-0406 Abstract The reactions between substituted isocyanates (RNCO) and other small molecules (e.g. water, alcohols, and amines) are of significant industrial importance, particularly for the development of novel polyurethanes and other useful polymers. We present very high level ab initio computations on the HNCO + H2O reaction, with results tar- geting the CCSDT(Q)/CBS//CCSD(T)/cc-pVQZ level of theory. Our results affirm that hydrolysis can occur across both the N−C and C−O bonds of HNCO via con- certed mechanisms to form carbamate or imidic acid with ∆H0K barrier heights of 38.5 −1 and 47.5 kcal mol . A total of 24 substituted RNCO + H2O reactions were studied. Geometries obtained with a composite method and refined with CCSD(T)/CBS single point energies determine that substituted RNCO species have a significant influence on these barrier heights, with an extreme case like fluorine lowering both barriers by close to 20 kcal mol−1 and most common alkyl substituents lowering both by approxi- mately 4 kcal mol−1. Natural Bond Oribtal (NBO) analysis provides evidence that the 1 predicted barrier heights are strongly associated with the occupation of the in-plane C−O* orbital of the RNCO reactant. -

United States Patent Office Faiented Vay 27, 1958 2 Substantially All of the Hydrogen Chioride Is Removed Therefrom
2,336,622 United States Patent Office Faiented Vay 27, 1958 2 substantially all of the hydrogen chioride is removed therefrom. With respect to the reactants usei in this process, the 2,336,622 phosgene car be preformed or it can be formed in situ PREPARATION OF CARBONY, FIUOR);E from carbon monoxide and chlorine which, as is weli Charles W. Tullock, Winnington, Dei., assig or its E. H. known, combine readily at elevated temperature to give iil Pont de Nemours and Conpany, Wii Saiigioia, Bei, phosgene. When this is done, the carbon monoxide is a corporation of Delaware preferably, though not necessarily, used in excess over the amount calculated to react with the chlorine. Simi No Drawing. Appication February 13, 1955 :) larly, the hydrogen fluoride can be used preformed, as the Seria No. 489,294 substantially anhydrous material, or if desired, it can be prepared in situ by employing approximately equivalent 10 Claims. (C. 260-544) amounts of a strong, substantially anhydrous acid such as sulfuric acid or hydrogen chloride and of an alkaline or This invention relates to a new process for preparing alkaline earth metal fluoride, such as Sodium or calcium fluorine-containing organic compounds. in particular, it fuoride. This embodiment is not preferred, however, be relates to a new process for preparing carbonyl fluoride. cause of the additional difficulties encountered in mixing It has recently been discovered that valuable fluorocar the reactants and in separating the reaction product from bons, including tetrafluoroethylene, can be synthesized in solid by-products. Another method of forming both very good yields by reacting carbonyl fluoride with car d phosgene and hydrogen fluoride in situ involves reacting bon at high temperatures, for exampie, by bringing car together formaldehyde, chlorine and a metal fluoride, bonyl fluoride in contact with the carbon electrodes of e.g., calcium fluoride.